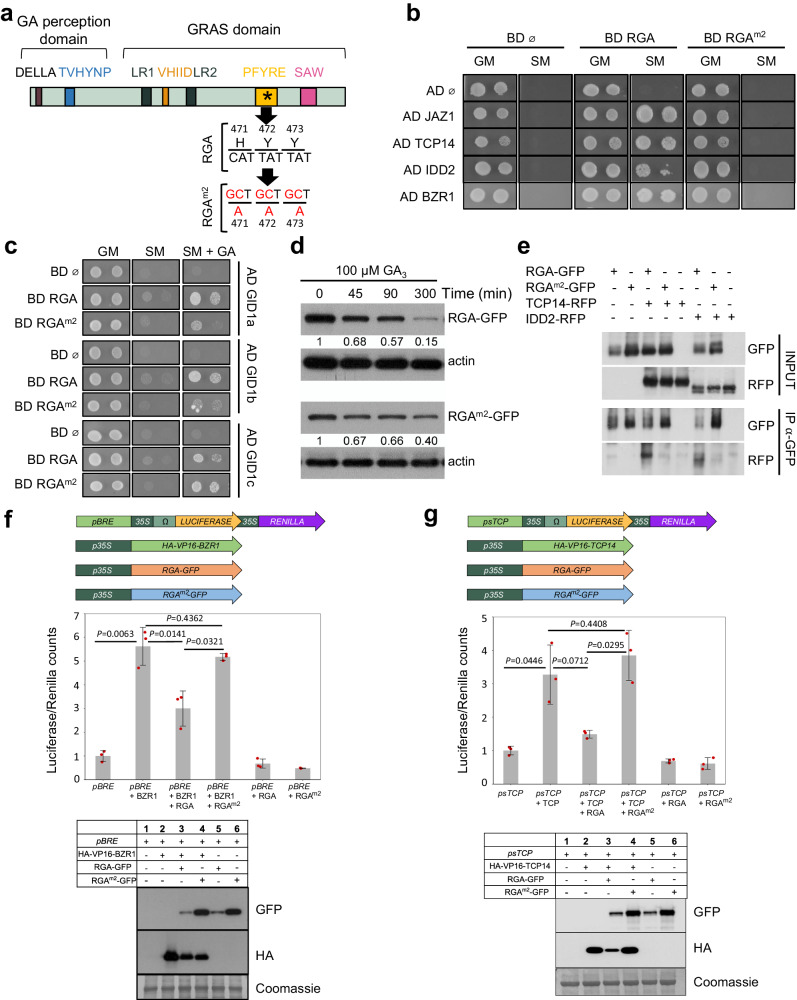
Fig. 1. The modified RGAm2 protein is an inactive DELLA that remains sensitive to GA.
a Schematic representation of the domain structure of a typical DELLA protein. Conserved histidine (H471), tyrosine (Y472), and tyrosine (Y473) residues were mutated into alanine (A) to obtain the modified RGAm2 protein. The nucleic acids and amino acids mutated in RGAm2 are indicated in red. b Yeast two-hybrid (Y2H) assays in which RGA and RGAm2 were tested pairwise with four known DELLA-interacting partners: JAZ1, TCP14, IDD2 and BZR1. Empty pGBKT7 and pGADT7 vectors were included as negative controls. Photos show the growth of the yeast on control media (GM) and on selective media (SM). c Pairwise Y2H interaction assays between RGA or RGAm2 and the three GA receptors GID1a, GID1b and GID1c. Photos show the growth of the yeast on control media (GM), selective media (SM), and SM media supplemented with 100 μM GA3. d Time-course analysis of GA-induced degradation of RGA (upper panel) and RGAm2 protein (lower panel). Immunodetection of RGA-GFP and RGAm2-GFP protein in 35S::RGA-GFP and 35S::RGAm2-GFP N. benthamiana agro-infiltrated leaves treated with 100 mM cycloheximide (CHX) and 100 µM GA3 for the indicated times. Numbers indicate RGA-GFP and RGAm2-GFP levels relative to actin levels, used as loading control. The experiment was repeated twice with similar results. e Co-immunoprecipitation assays between RGA or RGAm2 and IDD2 or TCP14. Protein extracts from different combinations of N. benthamiana agro-infiltrated leaves with 35S::RGA-GFP, 35 S::RGAm2-GFP, 35S::IDD2-RFP, and 35S::TCP14-RFP were immunoprecipitated with anti-GFP antibodies. The co-immunoprecipitated protein (IDD2-RFP and TCP14-RFP) was detected by anti-RFP antibodies. The experiment was repeated twice with similar results. f, g Effect of RGA and RGAm2 on BZR1 (f) and TCP14 (g) transcriptional activities in N. benthamiana agro-infiltrated leaves with a combination of BZR1, TCP14, RGA, and RGAm2 effector constructs and corresponding Luciferase/Renilla reporter constructs, as indicated (top panels). BZR1 and TCP14 have been fused to VP16 transcriptional activator domain in this experiment. Transcriptional activities are represented as the ratio of Luciferase and Renilla (used as internal control) activities, relative to the value obtained for the reporter construct alone that was set to 1. Data are means ± SD of three biological replicates. P-values were calculated in R using a two-tailed Welch t-test. Bottom panels: immunodetection of RGA-GFP, RGAm2-GFP, HA-VP16-BZR1, and HA-VP16-TCP14 from N. benthamiana agro-infiltrated leaves used for transcriptional activity assays. These experiments were repeated three times with similar results.