Abstract
Background
Sotorasib has been approved for the treatment of adult patients with KRAS G12C-mutated locally advanced or metastatic non-small cell lung cancer (NSCLC). Due to the limitations of clinical trials, potential adverse events (AEs) and long-term safety issues cannot be detected. The presented study aimed to evaluate sotorasib-associated AEs using the FDA Adverse Event Reporting System (FAERS) database.
Methods
Post-marketing AE reports of sotorasib in the database were collected for analysis. Disproportionality analyses, including the reporting odds ratio (ROR), proportional reporting ratio (PRR), information component (IC) and empirical bayes geometric mean (EBGM) algorithms, were performed to mine the signals of sotorasib-associated AEs. The median duration, quartiles and the Weibull shape parameter (WSP) test were used to assess the onset time data.
Results
The database contained 1538 cases of sotorasib as primary suspect (PS), with 27 signals detected, scattering in 5 SOCs. The SOC of hepatobiliary disorders (182, ROR 4.48, PRR 4.07, IC 2.02, EBGM 4.07) met the four methodological thresholds. The median onset time of sotorasib-associated AEs was 42 days (interquartile range [IQR] 14–86.75 days). Different SOCs had different types of risk over time.
Conclusion
After obtaining marketing authorization, the study identified all potentially relevant adverse event (AE) signals expected to have a reporting frequency higher than anticipated and characterized them during sotorasib treatment.
Keywords: Sotorasib, FAERS, AEs, Disproportionality analyses, WSP
1. Introduction
Non-small cell lung cancers (NSCLCs) represent 85 % of lung tumors [1]. The KRAS p. G12C mutation occurs in 13 % of NSCLCs and in 1–3 % of colorectal cancers and other cancers [2]. Local advanced or metastatic NSCLC with KRAS G12C-mutation accounts for approximately 14 % of advanced NSCLC cases [3]. Sotorasib (also known as AMG 510) is the world's first-in-class KRAS G12C mutation inhibitor and has been received accelerated approval from FDA on May 28, 2021. Due to the irreversible covalent bond formed between sotolasib and the cysteine of KRAS G12C [3], KRAS signaling is blocked, cell and tumor growth are inhibited, and cell apoptosis is only promoted in the KRAS G12C tumor cell line [4]. It is used to treat adult patients of locally advanced or metastatic NSCLC with KRAS G12C-mutation who have received at least one previous systemic treatment [3,5].
FDA's accelerated approval of sotorasib for marketing is based on the results of a Phase I/II clinical study codenamed CodeBreaK 100 [5]. In this experiment, researchers recruited 124 advanced NSCLC patients with KRAS G12C-mutation, 96 % of the participants had received at least two or more therapies including chemotherapy and immunotherapy. After sotorasib treatment, the drug resistance markers (hematopoietic factor receptor like kinases JAK1 and JAK2) significantly decreased, tumor growth was effectively inhibited with significant shrinkage. The overall response rate (ORR) of 36 % [95 % confidence interval (CI), 28–45] was achieved and median duration of response was 10.0 months (95 % CI, 6.9-not estimable). Among them, 58 % of patients experienced continuous response for no less than 6 months. A randomized, double-blind phase 3 clinical trial conducted in 148 centers across 22 countries showed that compared to docetaxel, sotorasib significantly increased progression free survival and improved safety in patients of advanced NSCLC with KRAS mutation [6]. Sotorasib is also used for the treatment of other advanced solid tumor patients with G12C mutation, including colorectal cancer, pancreatic cancer, endometrial cancer, appendix cancer and refractory colorectal cancer (combination therapy with panitumumab) [2,[7], [8], [9]].
According to the product description [4], the most common adverse drug reactions (ADRs) (≥20 %) were diarrhea, nausea, fatigue, musculoskeletal pain, hepatotoxicity and cough. The most common laboratory abnormalities (≥25 %) are decreased hemoglobin or lymphocytes, elevated aspartate aminotransferase or alanine aminotransferase, decreased calcium or sodium, elevated alkaline phosphatase or urinary protein. About 5 % of patients reduced the dose of sotorasib due to an adverse reaction, 34 % of patients interrupted dosage due to an adverse reaction. ≥ 2 % of patients were required dosage interruption because of AEs such as hepatotoxicity (11 %), diarrhea (8 %), musculoskeletal pain (3.9 %), nausea (2.9 %) and pneumonia (2.5 %), respectively. Permanent discontinuation in 9 % of patients due to AEs including hepatotoxicity [4].
Although several clinical trials have reported sotorasib related ADRs [[10], [11], [12]], due to the limitations of clinical trials, potential adverse events (AEs) and long-term safety issues cannot be detected. The FDA Adverse Event Reporting System (FAERS) design to support the post-market monitoring program for drugs and therapeutic bioproducts, all adverse event information and medication error information were collected including but not limited to drug side effects, drug abuse and misuse and drug interactions, etc. By conducting a comprehensive analysis of the FAERS database, our study aims to detect and analyze the AEs associated with sotorasib post-market, thereby enhancing our understanding of treatment outcomes, particularly AEs, in diverse patient populations and optimizing its application strategies.
2. Methods
2.1. Study design and data sources
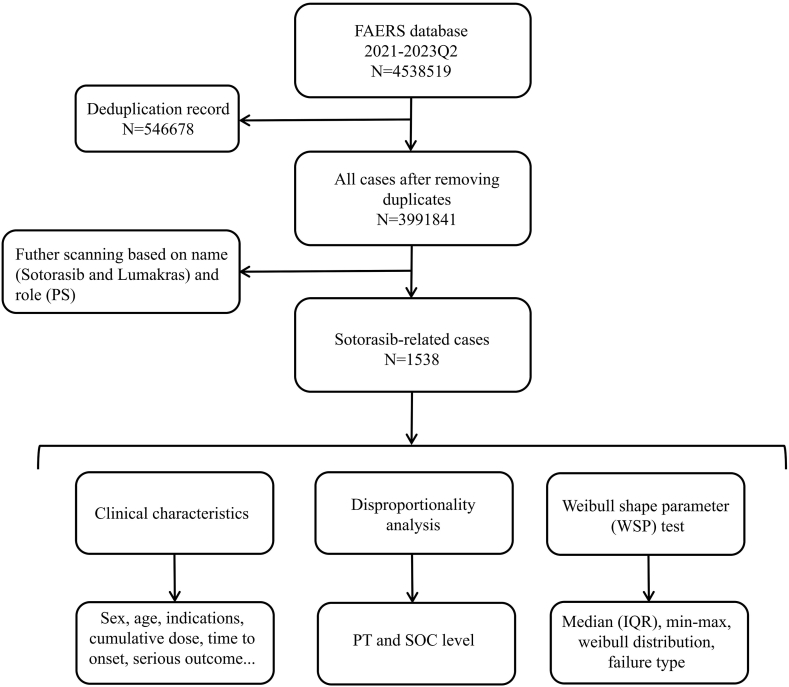
The study was designed as a case/non-case study, and a total of 10 quarterly data in FAERS were screened covering the period from January 2021 to June 2023. The FAERS data files comprised seven types of datasets: patient demographic and administrative information (DEMO), drug/biologic information (DRUG), adverse events (REAC), patient outcomes (OUTC), report sources (RPSR), start and end dates of drug therapy (THER), and indications for use/diagnosis (INDI) [13]. The unique identification number for each report was used to link seven files. A total of 4538519 reports were retrieved during the study period. Due to the existence of a large number of duplicated reports, we removed the redundant records before performing statistical analysis, based on the PRIMARYID and CASEID column in the DEMO file as previously described [14,15], reducing the data to 3991841 (Fig. 1). Futher scanning were performed based on the brand name (lumakras) and generic name (sotorasib) approved by FDA. Moreover, only the reports with primary suspect (PS) role in the DRUG file were selected to ensure results with high relevancy. AEs in FAERS were coded using the preferred term (PT) codes by standardized Medical Dictionary for Regulatory Activities (MedDRA®, version 26.1) [15], which has a hierarchical structure and allows PT grouping at different levels (high level term [HLT], high level group term [HLGT], system organ class [SOC]).
Fig. 1.
Multi-step process of data extraction, processing, and analysis for sotorasib-related cases.
2.2. Data mining
Descriptive analyses were performed to collect as many clinical characteristics as possible for all sotorasib-associated reports. Because many items were not required fields in the database, we considered the effect of unexploitable data and calculated the available data, such as sex, age, weight, indications, cumulative dose, outcomes, combination medication and reporting countries, etc. Disproportionality analyses, including reporting odds ratio (ROR), proportional reporting ratio (PRR), the information component (IC) and the empirical bayes geometric mean (EBGM), are used to identify drug-associated AEs (signals) that are reported more frequently than expected by estimating proportion of specific AEs occurrence between a specific drug and all other drugs [16,17]. We performed the disproportionality analyses at both PT and SOC levels to explore the correlation of sotorasib at different hierarchies. The associations between drugs and AEs were measured by the values for the four calculation methods (Table 1). When multiple algorithm standards are consistent, the analysis results are more reliable, and AE was considered overreporting when four algorithmic criteria were met simultaneously [18].
Table 1.
Four algorithms used to assess potential associations between sotorasib and AEs.
| Algorithms | Equation | Criteria |
|---|---|---|
| ROR | ROR = ad/b/c | lower limit of 95 % CI > 1, N ≥ 3 |
| 95%CI = eln(ROR)±1.96(1/a+1/b+1/c+1/d)^0.5 | ||
| PRR | PRR = a(c + d)/c/(a+b) | PRR≥2, χ2 ≥ 4, N ≥ 3 |
| χ2 = [(ad-bc)^2](a+b + c + d)/[(a+b) (c + d) (a+c) (b + d)] | ||
| BCPNN | IC = log2a(a+b + c + d)/((a+c) (a+b)) | IC025 > 0 |
| 95%CI = E(IC) ± 2 V(IC)^0.5 | ||
| MGPS | EBGM = a(a+b + c + d)/(a+c)/(a+b) | EBGM05 > 2 |
| 95%CI = eln(EBGM)±1.96(1/a+1/b+1/c+1/d)^0.5 |
Equation: a, number of reports containing both the target drug and target AE; b, number of reports containing other AE of the target drug; c, number of reports containing the target AE of other drugs; d, number of reports containing other drugs and other AE. AE, adverse event; 95%CI, 95 % confidence interval; N, the number of reports; χ2, chi-squared; IC, information component; IC025, the lower limit of 95 % CI of the IC; E(IC), the IC expectations; V(IC), the variance of IC; EBGM, empirical Bayesian geometric mean; EBGM05, the lower limit of 95 % CI of EBGM.
2.3. Time to onset
Time to onset (TTO) was calculated from the initiation of sotorasib treatment to the occurrence of the AEs related to sotorasib. Data errors or missing were removed, and only reports for which TTO data were available were analyzed. The median duration, quartiles and the Weibull shape parameter (WSP) test were used to assess the TTO data. The incidence of AEs depends on the mechanism of drug action and often fluctuates with the duration of treatment. The WSP test is used for statistical analysis of TTO and describes the risk of AE increasing or decreasing over time [19]. The characteristics of the Weibull distribution are described by the scale parameter (α) and the shape parameter (β). The evaluation criterion for the selected parameter were detailed in previous studies [20]. After initiation of sotorasib treatment, the median TTO and WSP of the different SOC signals were calculated to predict the hazard of these AEs occurring over time in SOC levels.
All data storage, filtering, processing and statistical analysis were performed by MySQL 8.0, R software 4.2, and Microsoft EXCEL 2019.
3. Results
3.1. Descriptive analysis
As of second quarter of 2023, a total of 1538 sotorasib-associated reports as PS were recorded in the FAERS database, among which contained 2748 AEs. The clinical characteristics of cases with sotorasib were detailedly described in Table 2. After sotorasib goes on sale, the number of AE reports increase significantly and rapidly. There were 146 (9.49 %), 629 (40.90 %), and 763 (49.61 %) sotorasib-associated reports received in 2021, 2022, and the first half of 2023, respectively. The proportion of females was slightly higher than that of males (53.42 % vs 46.58 %). The proportion of elderly patients was higher (≥65 years, 61.07 %), with a median age of 68 (interquartile range [IQR] 61–74) years. The median weight of the patients was 64.1 years (IQR 52–75.2, with data available in only 173 case reports, 11.25 %). The most reported indication of sotorasib was lung cancer (89.72 %, valid reports in 890/992) and intestinal cancer (3.93 %, valid reports in 39/992). The median cumulative dose at onset was 47.04 g (IQR 21.12–66.24, with only 107 case reports, 6.96 %). Additionally, valid onset time was recorded in 28.74 % of the reports, with the median TTO of 42 days (IQR 14–86.75 days). A total of 70.61 % of the reports had a serious outcome, including 336 deaths (30.94 %). Of note, deaths were more likely to be related to disease progression than to sotorasib-associated AEs. Among sotorasib-induced reports, 360 cases (23.41 %) were presented in combination with other drugs. The top five combination medications were acetaminophen (16.67 %), folic acid (10.83 %), gabapentin (10.56 %), atorvastatin (9.44 %), and dexamethasone (9.17 %). More than half of the reports came from the United States (51.43 %). Most of the reports were submitted by physician (55.74 %), followed by consumer (20.15 %), health-professional (14.57 %) and pharmacist (9.54 %).
Table 2.
Clinical characteristics of reports with sotorasib from the FAERS database (2021–2023 Q2).
| Characteristics | Available case number, n | Case proportion, % |
|---|---|---|
| Number of case | 1538 | 100 |
| Sex | 1142 | 74.25 |
| Female | 610 | 53.42 |
| Male | 532 | 46.58 |
| Age (year) | 655 | 42.59 |
| <65 | 255 | 38.93 |
| ≥65 | 400 | 61.07 |
| IQR | 68 (61–74) | / |
| Weight (kg) | 173 | 11.25 |
| <70 | 109 | 63.01 |
| ≥70 | 64 | 36.99 |
| IQR | 64.1 (52–75.2) | / |
| Indications | 992 | 64.50 |
| Lung cancer | 890 | 89.72 |
| Intestinal cancer | 39 | 3.93 |
| Cumulative dose (g) | 107 | 6.96 |
| <50 | 59 | 55.14 |
| ≥50 | 48 | 44.86 |
| IQR | 47.04 (21.12–66.24) | / |
| Time to onset (day) | 442 | 28.74 |
| ≤30 | 189 | 42.76 |
| >30 | 253 | 57.24 |
| IQR | 42 (14–86.75) | / |
| Serious outcome | 1086 | 70.61 |
| Death (DE) | 336 | 30.94 |
| Life-threatening (LT) | 19 | 1.75 |
| Hospitalization (HO) | 285 | 26.24 |
| Disability (DS) | 7 | 0.64 |
| Congenital anomaly (CA) | 22 | 2.03 |
| Required intervention to prevent permanent impairment/damage (RI) | 1 | 0.09 |
| Other serious medical events (OT) | 788 | 71.64 |
| Combination medication (Top five) | 360 | 23.41 |
| Acetaminophen | 60 | 16.67 |
| Folic acid | 39 | 10.83 |
| Gabapentin | 38 | 10.56 |
| Atorvastatin | 34 | 9.44 |
| Dexamethasone | 33 | 9.17 |
| Reported countries (Top five) | 1538 | 100 |
| America (US) | 791 | 51.43 |
| France (FR) | 257 | 16.71 |
| Japan (JP) | 185 | 12.03 |
| Germany (DE) | 52 | 3.38 |
| Italy (IT) | 43 | 2.80 |
| Reporter type | 1489 | 96.81 |
| Physician (MD) | 830 | 55.74 |
| Pharmacist (PH) | 142 | 9.54 |
| Health-professional (HP) | 217 | 14.57 |
| Consumer (CN) | 300 | 20.15 |
| Reporting year | 1538 | 100 |
| 2023 Q2 | 763 | 49.61 |
| 2022 | 629 | 40.90 |
| 2021 | 146 | 9.49 |
IQR, interquartile range; Q2, the second quarter.
3.2. Disproportionality analysis
The signal strength and number of reports of sotorasib at the SOC level were presented in Table 3. In general, we found that all sotorasib-associated AEs were concentrated in 26 organ systems. The strength of SOC simultaneously satisfying the four methodological thresholds was hepatobiliary disorders (n = 182, ROR 4.48 [3.84–5.23], PRR 4.07 [433.49], IC 2.02 [1.77], EBGM 4.07 [3.48]). In order to get more SOCs that are worth considering safety for broader clinical applications of sotorasib, the SOCs conforming to at least one of these methodologies were also included respiratory, thoracic and mediastinal disorders (n = 444, ROR 2.25 [2.02–2.51], PRR 1.89 [219.4], IC 0.92 [0.76], EBGM 1.89 [1.69]) and neoplasms benign, malignant and unspecified (n = 380, ROR 2.09 [1.86–2.35], PRR 1.82 [162.5], IC 0.86 [0.69], EBGM 1.82 [1.62]).
Table 3.
Signal strength of reports of sotorasib at the System Organ Class (SOC) level in FAERS database.
| System Organ Class (SOC) | Cases | ROR (95 % two-sided CI) | PRR (χ2) | IC (IC025) | EBGM (EBGM05) |
|---|---|---|---|---|---|
| General disorders and administration site conditions | 576 | 0.95 (0.86–1.05) | 0.97 (1.01) | −0.05 (−0.18) | 0.97 (0.87) |
| Respiratory, thoracic and mediastinal disorders | 444 | 2.25 (2.02–2.51)a | 1.89 (219.4) | 0.92 (0.76)a | 1.89 (1.69) |
| Neoplasms benign, malignant and unspecified | 380 | 2.09 (1.86–2.35)a | 1.82 (162.5) | 0.86 (0.69)a | 1.82 (1.62) |
| Gastrointestinal disorders | 312 | 1.13 (0.99–1.27) | 1.10 (3.48) | 0.14 (−0.04) | 1.10 (0.97) |
| Injury, poisoning and procedural complications | 217 | 0.37 (0.32–0.42) | 0.46 (202.81) | −1.13 (−1.34) | 0.46 (0.40) |
| Hepatobiliary disorders | 182 | 4.48 (3.84–5.23)a | 4.07 (433.49)a | 2.02 (1.77)a | 4.07 (3.48)a |
| Investigations | 178 | 1.02 (0.87–1.19) | 1.02 (0.05) | 0.02 (−0.21) | 1.02 (0.87) |
| Nervous system disorders | 112 | 0.35 (0.28–0.42) | 0.39 (128.8) | −1.35 (−1.63) | 0.39 (0.32) |
| Metabolism and nutrition disorders | 98 | 0.97 (0.79–1.19) | 0.97 (0.07) | −0.04 (−0.35) | 0.97 (0.79) |
| Cardiac disorders | 90 | 0.55 (0.45–0.68) | 0.58 (30.91) | −0.79 (−1.11) | 0.58 (0.47) |
| Musculoskeletal and connective tissue disorders | 90 | 0.46 (0.38–0.57) | 0.50 (52.35) | −1.01 (−1.33) | 0.50 (0.40) |
| Vascular disorders | 80 | 0.35 (0.28–0.44) | 0.39 (89.4) | −1.37 (−1.70) | 0.39 (0.31) |
| Infections and infestations | 64 | 0.31 (0.24–0.39) | 0.34 (96.44) | −1.58 (−1.95) | 0.34 (0.26) |
| Skin and subcutaneous tissue disorders | 52 | 0.21 (0.16–0.28) | 0.24 (148.34) | −2.07 (−2.49) | 0.24 (0.18) |
| Psychiatric disorders | 49 | 0.22 (0.17–0.30) | 0.25 (129.35) | −2.02 (−2.44) | 0.25 (0.19) |
| Surgical and medical procedures | 48 | 0.81 (0.61–1.08) | 0.81 (2.10) | −0.30 (−0.74) | 0.81 (0.61) |
| Blood and lymphatic system disorders | 43 | 0.51 (0.38–0.70) | 0.53 (19.20) | −0.92 (−1.39) | 0.53 (0.39) |
| Renal and urinary disorders | 32 | 0.26 (0.19–0.38) | 0.28 (64.10) | −1.84 (−2.36) | 0.28 (0.20) |
| Immune system disorders | 27 | 0.16 (0.11–0.24) | 0.18 (112.18) | −2.48 (−3.05) | 0.18 (0.12) |
| Endocrine disorders | 10 | 0.28 (0.15–0.52) | 0.28 (18.68) | −1.82 (−2.78) | 0.28 (0.15) |
| Eye disorders | 7 | 0.10 (0.05–0.22) | 0.11 (53.73) | −3.21 (−4.32) | 0.11 (0.05) |
| Product issues | 7 | 0.09 (0.04–0.19) | 0.09 (64.41) | −3.41 (−4.53) | 0.09 (0.04) |
| Reproductive system and breast disorders | 6 | 0.06 (0.03–0.13) | 0.06 (91.59) | −4.02 (−5.22) | 0.06 (0.03) |
| Congenital, familial and genetic disorders | 3 | 0.38 (0.12–1.17) | 0.38 (3.10) | −1.41 (−3.25) | 0.38 (0.12) |
| Ear and labyrinth disorders | 1 | 0.05 (0.01–0.38) | 0.05 (16.73) | −4.21 (−7.17) | 0.05 (0.01) |
| Pregnancy, puerperium and perinatal conditions | 1 | 0.04 (0.01–0.26) | 0.04 (24.87) | −4.72 (−7.66) | 0.04 (0.01) |
indicates statistically significant signals in algorithm. ROR, reporting odds ratio; CI, confidence interval; PRR, proportional reporting ratio; χ2, chi-squared; IC, information component; IC025, the lower limit of 95 % CI of the IC; EBGM, empirical Bayesian geometric mean; EBGM05, the lower limit of 95 % CI of EBGM.
After excluding PTs caused by non-drug therapy, a total of 27 sotorasib-related AE signals were screened by four algorithms, which were scattered in 5 SOCs (Table 4), including 12 PTs in hepatobiliary disorders, 9 PTs in investigations, 3 PTs in gastrointestinal disorders, 2 PTs in respiratory, thoracic and mediastinal disorders, and 1 PT in cardiac disorders. Moreover, in order to mine more suspicious signals, we also listed the additional signals in Table 5 that only satisfy the ROR methodology, with the results of 15 PTs in 6 SOCs.
Table 4.
Signal strength of reports of sotorasib at the Preferred Term (PT) level in FAERS database.
| SOC | Preferred Terms (PTs) | Cases | ROR (95 % two-sided CI) | PRR (χ2) | IC (IC025) | EBGM (EBGM05) |
|---|---|---|---|---|---|---|
| Hepatobiliary disorders | Hepatotoxicity | 41 | 22.99 (16.84–31.39) | 22.40 (832.21) | 3.85 (3.39) | 22.22 (16.27) |
| Hepatic function abnormal | 25 | 9.73 (6.55–14.46) | 9.59 (191.96) | 2.79 (2.21) | 9.56 (6.43) | |
| Liver disorder | 19 | 6.55 (4.16–10.30) | 6.48 (88.00) | 2.27 (1.60) | 6.47 (4.11) | |
| Hepatic cytolysis | 17 | 9.97 (6.18–16.10) | 9.87 (135.18) | 2.64 (1.93) | 9.84 (6.09) | |
| Cholestasis | 14 | 12.12 (7.15–20.55) | 12.02 (140.95) | 2.69 (1.91) | 11.97 (7.07) | |
| Hepatitis | 9 | 6.31 (3.27–12.16) | 6.28 (39.88) | 1.89 (0.92) | 6.27 (3.25) | |
| Jaundice | 8 | 7.33 (3.65–14.70) | 7.30 (43.37) | 1.93 (0.91) | 7.28 (3.63) | |
| Hepatic failure | 7 | 4.60 (2.19–9.67) | 4.59 (19.61) | 1.47 (0.37) | 4.58 (2.18) | |
| Hypertransaminasaemia | 6 | 9.67 (4.33–21.58) | 9.63 (46.27) | 1.88 (0.70) | 9.60 (4.30) | |
| Cholangitis | 4 | 9.92 (3.71–26.50) | 9.89 (31.86) | 1.51 (0.06) | 9.86 (3.69) | |
| Hepatitis cholestatic | 4 | 13.30 (4.97–35.58) | 13.27 (45.16) | 1.62 (0.17) | 13.21 (4.94) | |
| Immune-mediated hepatitis | 4 | 17.69 (6.61–47.36) | 17.65 (62.41) | 1.70 (0.25) | 17.54 (6.55) | |
| Investigations | Alanine aminotransferase increased | 33 | 10.46 (7.40–14.78) | 10.26 (275.24) | 2.96 (2.46) | 10.22 (7.24) |
| Liver function test increased | 32 | 18.63 (13.11–26.48) | 18.27 (519.24) | 3.53 (3.02) | 18.15 (12.77) | |
| Aspartate aminotransferase increased | 31 | 11.86 (8.31–16.94) | 11.64 (300.82) | 3.08 (2.55) | 11.60 (8.12) | |
| Hepatic enzyme increased | 29 | 5.87 (4.06–8.48) | 5.78 (114.70) | 2.27 (1.72) | 5.77 (3.99) | |
| Blood alkaline phosphatase increased | 13 | 13.14 (7.60–22.71) | 13.04 (143.86) | 2.70 (1.89) | 12.98 (7.51) | |
| Blood bilirubin increased | 10 | 7.62 (4.09–14.20) | 7.58 (56.95) | 2.11 (1.19) | 7.56 (4.05) | |
| Gamma-glutamyltransferase increased | 7 | 7.00 (3.33–14.73) | 6.97 (35.75) | 1.80 (0.71) | 6.96 (3.31) | |
| Liver function test abnormal | 7 | 7.48 (3.56–15.73) | 7.45 (39.00) | 1.85 (0.76) | 7.43 (3.53) | |
| Transaminases increased | 7 | 4.91 (2.34–10.33) | 4.89 (21.67) | 1.52 (0.43) | 4.89 (2.32) | |
| Respiratory, thoracic and mediastinal disorders | Pneumonitis | 14 | 7.04 (4.16–11.92) | 6.99 (71.70) | 2.22 (1.44) | 6.97 (4.11) |
| Pleural effusion | 13 | 3.75 (2.17–6.48) | 3.73 (25.99) | 1.53 (0.73) | 3.73 (2.16) | |
| Gastrointestinal disorders | Diarrhea | 163 | 3.76 (3.19–4.42) | 3.47 (294.63) | 1.76 (1.52) | 3.46 (2.94) |
| Colitis | 11 | 3.90 (2.16–7.07) | 3.88 (23.56) | 1.52 (0.65) | 3.88 (2.14) | |
| Gastrointestinal toxicity | 7 | 20.11 (9.54–42.38) | 20.02 (125.57) | 2.37 (1.27) | 19.88 (9.43) | |
| Cardiac disorders | Pericardial effusion | 8 | 5.37 (2.68–10.76) | 5.35 (28.24) | 1.68 (0.65) | 5.34 (2.66) |
ROR, reporting odds ratio; CI, confidence interval; PRR, proportional reporting ratio; χ2, chi-squared; IC, information component; EBGM, empirical Bayesian geometric mean.
Table 5.
Supplementary signals that merely satisfy the threshold of the ROR method.
| SOC | Preferred Terms (PTs) | Cases | ROR (95 % two-sided CI) | PRR (χ2) | IC (IC025) | EBGM (EBGM05) |
|---|---|---|---|---|---|---|
| Metabolism and nutrition disorders | Decreased appetite | 29 | 1.75 (1.21–2.53) | 1.74 (9.22) | 0.71 (0.17) | 1.74 (1.20) |
| Hypocalcaemia | 4 | 3.41 (1.28–9.09) | 3.40 (6.77) | 0.88 (−0.57) | 3.40 (1.27) | |
| Hepatobiliary disorders | Drug-induced liver injury | 8 | 3.18 (1.59–6.37) | 3.17 (11.86) | 1.18 (0.16) | 3.16 (1.58) |
| Acute hepatic failure | 3 | 3.46 (1.12–10.76) | 3.46 (5.24) | 0.68 (−0.99) | 3.46 (1.11) | |
| Cholestatic liver injury | 3 | 16.77 (5.38–52.24) | 16.74 (44.12) | 1.35 (−0.33) | 16.64 (5.34) | |
| Mixed liver injury | 3 | 11.22 (3.61–34.91) | 11.20 (27.75) | 1.24 (−0.43) | 11.16 (3.59) | |
| Investigations | Blood bilirubin abnormal | 3 | 33.47 (10.7–104.65) | 33.41 (93.11) | 1.46 (−0.22) | 32.99 (10.55) |
| Blood magnesium decreased | 3 | 4.67 (1.50–14.51) | 4.66 (8.62) | 0.87 (−0.80) | 4.66 (1.50) | |
| Hepatic enzyme abnormal | 3 | 8.27 (2.66–25.71) | 8.25 (19.07) | 1.14 (−0.53) | 8.23 (2.65) | |
| Musculoskeletal and connective tissue disorders | Myalgia | 17 | 1.79 (1.11–2.90) | 1.79 (5.91) | 0.69 (−0.01) | 1.79 (1.11) |
| Respiratory, thoracic and mediastinal disorders | Pulmonary embolism | 13 | 2.81 (1.63–4.85) | 2.79 (14.97) | 1.20 (0.40) | 2.79 (1.62) |
| Acute respiratory failure | 5 | 3.48 (1.45–8.39) | 3.48 (8.81) | 1.04 (−0.26) | 3.47 (1.44) | |
| Lung opacity | 3 | 7.96 (2.56–24.76) | 7.95 (18.18) | 1.12 (−0.55) | 7.93 (2.55) | |
| Pulmonary toxicity | 3 | 5.11 (1.65–15.89) | 5.10 (9.88) | 0.92 (−0.75) | 5.10 (1.64) | |
| General disorders and administration site conditions | Oedema | 7 | 2.36 (1.12–4.97) | 2.36 (5.48) | 0.82 (−0.28) | 2.36 (1.12) |
ROR, reporting odds ratio; CI, confidence interval; PRR, proportional reporting ratio; χ2, chi-squared; IC, information component; EBGM, empirical Bayesian geometric mean.
3.3. Time to onset analysis
Results of TTO analysis for overall 42 (IQR 14–86.75) days, hepatobiliary disorders 39 (IQR 23–54) days, investigations 25 (IQR 14–44) days, respiratory, thoracic and mediastinal disorders 29 (IQR 7–69.5) days, gastrointestinal disorders 18 (IQR 7–53) days and cardiac disorders 23 (9–56) days were summarized in Table 6. Most of the cases occurred within the first1 (n = 189, 42.76 %), 2 (n = 281, 63.57 %) and 3 months (n = 337, 76.24 %) after sotorasib initiation. In the WSP analysis, both the shape parameter β and the upper limit of its 95 % CI were <1, suggesting overall, respiratory, thoracic and mediastinal disorders, gastrointestinal disorders and cardiac disorders had early failure types, with the hazard of occurrence gradually decreased over time. However, since the shape parameter β is equal to or nearly 1 and its 95 % CI contained the value 1, the hepatobiliary disorders and investigations were random failure types, with the hazard of continuous occurrence over time. Furthermore, the cumulative incidence over time in different scenarios were plotted in Fig. 2.
Table 6.
The results of time to onset analysis for signals with different SOCs.
| SOC | TTO (days) |
Weibull distribution |
Failure type | |||||
|---|---|---|---|---|---|---|---|---|
| Cases |
Parameter |
Scale parameter |
Shape parameter |
|||||
| n | Median (IQR) | Min-max | α | 95 % CI | β | 95 % CI | ||
| Overall | 442 | 42 (14–86.75) | 0–525 | 57.91 | 49.91–67.19 | 0.65 | 0.60–0.70 | Early failure |
| Hepatobiliary disorders | 85 | 39 (23–54) | 2–376 | 52.27 | 42.40–64.43 | 1.08 | 0.93–1.25 | Random failure |
| Investigations | 59 | 25 (14–44) | 0–307 | 36.04 | 26.90–48.28 | 0.91 | 0.76–1.10 | Random failure |
| Respiratory, thoracic and mediastinal disorders | 51 | 29 (7–69.5) | 0–264 | 44.93 | 30.32–66.59 | 0.73 | 0.58–0.91 | Early failure |
| Gastrointestinal disorders | 85 | 18 (7–53) | 0–525 | 29.83 | 20.74–42.89 | 0.61 | 0.51–0.72 | Early failure |
| Cardiac disorders | 19 | 23 (9–56) | 0–437 | 40.38 | 17.70–92.13 | 0.57 | 0.40–0.81 | Early failure |
n, number of cases with available time-to-onset; IQR, interquartile range; TTO, Time-to-onset. When TTO, is 0 days, the adverse event occurred within the same day with the therapy.
Fig. 2.
Cumulative distribution curves of the onset time.
4. Discussion
To the best of our knowledge, this is the first post-marketing pharmacovigilance study of sotorasib-associated AEs based on the FAERS database. Disproportionality analyses were performed to mine the signals of sotorasib-associated AEs. More than half of the reports came from the United States (51.43 %), followed by France (16.71 %) and Japan (12.03 %). From global epidemiology of lung cancer [21], the incidence rate in American women is now higher than that in men. In Europe, the morbidity of female is still rising (except the United Kingdom, Denmark and the Netherlands). In Asia, the female morbidity in China, Japan and South Korea is also rising. The morbidity of male in other European countries is declining (except France and Spain, where it is basically stable). The slightly higher proportion of females (53.42 %) compared to males (46.58 %) in our study may be attributed to this factor.
In the 2-year analysis of CodeBreaK 100, which is the largest clinical data set with the longest follow-up reported for patients treated with KRAS G12C inhibitor [11], sotorasib treatment-related any-grade AEs have been observed in 70 % of patients received sotorasib 960 mg once daily, with grade 3 and grade 4 in 20 % and 1 %, and no fatal AEs [11]. Among them 22 % and 6 % were led to treatment reduction or interruption and discontinuation, which were lower than that in the product description [4]. In our results, serious outcome including life-threatening, hospitalization, disability and other serious medical events accounting for 70.61 % were observed in 1538 sotorasib-associated reports, which was higher than that in the 2-year analysis of CodeBreaK 100 with 174 patients [11]. Although variations in the definition and attribution of serious outcomes may exist, and it may not reliably identify significant differences within the real population, but can provide some reference for clinical practitioners.
In our study, most of the cases occurred within the 1 (n = 189, 42.76 %), 2 (n = 281, 63.57 %) and 3 months (n = 337, 76.24 %) after sotorasib initiation. Valid onset time was recorded in 28.74 % of the reports, with the median TTO of 42 days (IQR 14–86.75 days), which consistent with the median (range) time to diarrhea and hepatotoxicity onset with 6.1 (1.7–11.1) and 9.1 (3.1–18.7) weeks in the clinical research report [11]. Interestingly, the median cumulative dose at onset was 47.04 g, which is approximately equivalent to a normal dose of 960 mg once daily (recommended dosage by FDA) for about 42 days.
Diarrhea (163 [10 %]), increased alanine aminotransferase (33 [2 %]), and increased aspartate aminotransferase (31 [2 %]) were found to be the most common AEs. Despite the absence of an exact denominator that could have resulted in lower values, they still remain inferior to those observed in the 2-year analysis of CodeBreaK 100 (30 %, 18 %, and 18 % respectively). However, compared to RCTs, the FAERS database not only lacks a denominator but also suffers from significant underreporting of adverse reactions, as this value would be even lower if there were data on the population using the medication. There have been reports of nervous system disorders, metabolism and nutrition disorders, cardiac disorders, musculoskeletal and connective tissue disorders, as well as vascular complications associated with sotorasib administration. This suggests that patients with pre-existing neurological or other systemic conditions should exercise caution regarding the potential impact of sotorasib on their underlying condition.
In addition to elevated levels of alanine aminotransferase and aspartate aminotransferase, adverse events associated with sotorasib included 8 cases of jaundice and 10 cases of increased blood bilirubin. A substantial number of adverse reactions associated with hepatotoxicity in the SOCs of hepatobiliary disorders and investigations indicate that caution should be exercised by clinicians when considering the use of sotorasib for treatment due to its potential hepatic toxicity. Retrospective analysis of clinical trials conducted across 16 medical centers in France revealed that the combination therapy involving sotorasib and anti-PD-(L)1 agents may potentially induce severe immune-mediated hepatotoxicity. Consequently, it is recommended to avoid initiating sotorasib treatment within a period of 30 days following the last infusion of anti-PD-(L)1 [22]. In addition, approximately 23.41 % (360 cases) of reported sotorasib-induced AEs were associated with concomitant use of other drugs in our study, with acetaminophen, folic acid, gabapentin, atorvastatin, and dexamethasone identified as the top five combination medications, accounting for 9.17 %–16.67 % of cases. Acetaminophen is a non steroidal anti-inflammatory drug that inhibits the synthesis of prostaglandins, which is widely used clinically for antipyretic and analgesic purposes. It is relatively safe at therapeutic doses, however, excessive acetaminophen may lead to fatal acute liver injury. Acetaminophen induced hepatotoxicity (AIH) is mainly caused by the toxic metabolite N-acetyl benzoquinone imine (NAPQI) [23]. Inflammation and oxidative stress play important roles in liver injury, dexamethasone induces oxidative stress and activation of caspase-3, leading to liver toxicity [24]. A study evaluating the relationship between atorvastatin and liver toxicity in a real-world environment showed that the risk of liver toxicity increased by 1.3–1.5 times after taking atorvastatin [25]. Thus when used in combination with sotorasib, it will be possible to increase the risk of hepatotoxicity. In addition, acetaminophen, dexamethasone or atorvastatin are P-gp substrates [[26], [27], [28]]. In the instruction manual of sotorasib, it is mentioned to avoid coadministration with P-gp substrates, as minimal concentration changes may lead to severe toxicities [4]. So if coadministration cannot be avoided, the substrate dose is suggested to be reduced based on its prescription information. Because sotorasib is primarily metabolized by CYP3A4, specific dose modification strategies are recommended for concomitant use of sotorasib with CYP3A4 inducers (oxcarbazepine, phenobarbital, honey, vitamins,etc.) and substrates (codeine, cyclosporin, diazepam and erythromycin, etc.), P-glycoprotein (P-gp) substrates (taxanes, tyrosine kinase inhibitors, and some PARP inhibitors, etc) and acid-reducing agents (proton pump inhibitors, H2 receptor blockers) [4]. It is noteworthy that nervous system disorders account for 7.28 % of sotorasib-associated reports in this study, which with few relevant research reports. Brain metastasis occurs in approximately 40 % of patients with KRAS mutant NSCLC [29], significantly affects the morbility and are associated with worse survival [30]. Therefore those nervous system disorders maybe relate to the brain metastasis in patients with KRASG12C-mutant instead of adverse reactions.
There are some limitations in our study. First, the reporter of adverse reactions may use self-report to obtain data, which may have issues with memory bias and subjective perception. Second, FAERS includes information submitted to the FDA regarding adverse events and reports of drug use errors, but it should be noted that the presence of reports in FAERS does not prove a causal relationship between AEs and drug itself, which needs to be validated through prospective clinical studies. Indeed, measures of disproportionality analysis cannot estimate risks or necessarily account for a causal association; rather, they only facilitate the identification of adverse events that are expected to have a higher reporting frequency than anticipated. The findings from disproportionality analysis require cautious interpretation, assessment of bias risk, and clinical evaluation (qualitative analysis) before drawing any causal inferences [31]. Third, although more possible AEs can be detected, some rare new AEs may also be overlooked. For sotorasib, hemolytic anemia as a grade 3 new-onset AE was found in 2 % of patients [11], but our study did not detect this adverse reaction by disproportionality analysis (only 2 cases). When adverse reactions occur, we need to comprehensively evaluate the patient's primary disease and its progression (such as brain metastasis, lymph node metastasis), drug/food interactions, time and dose correlation of symptom occurrence, and other factors.
5. Conclusion
Based on FAERS database, the study comprehensively and systematically revealed the AE signals and time to AEs onsets in treatment with sotorasib. We unearthed 27 sotorasib-associated signals, and the SOC of hepatobiliary disorders was the main pathogenic organ. The median cumulative dose at onset was 47.04 g, and the median TTO was 42 days (IQR 14–86.75 days). In addition, the WSP test showed more characteristics of TTO data. In addition, we explored the potential impact of underlying diseases and drug interactions in the clinical application of sotorasib, as well as the need to pay attention to adjusting the drug dosage when used in combination with anti-PD - (L) 1 agents, P-gp substrates or CYP3A4 inducers, etc. Given the increasing use of sotorasib, pharmacovigilance studies may play an important role in facilitating risk-benefit assessment through large real-world databases, especially for unanticipated AEs that are not documented by the label. In conclusion, our findings and management recommendations may improve clinicians/researchers awareness of sotorasib-associated toxicity and help reduce risk.
Ethics declarations
Review and/or approval by an ethics committee was not needed for this study because no patients participate in the study. Informed consent was not required for this study because no patients participate in the study and no anonymised case details and images were publicated.
Data availability statement
The data included in article/supplementary/referenced in article.
CRediT authorship contribution statement
Yiling Ding: Writing – review & editing, Writing – original draft, Software, Methodology, Formal analysis, Data curation, Conceptualization. Hongyan Su: Software, Methodology, Formal analysis, Data curation, Conceptualization. Yamin Shu: Writing – review & editing, Validation, Investigation, Data curation. Jing Chen: Writing – review & editing, Writing – original draft, Visualization, Software, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was supported by grants from Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (No. 2022B26).
References
- 1.Meshulami N., Tavolacci S., de Miguel-Perez D., Rolfo C., Mack P.C., Hirsch F.R. Predictive Capability of PD-L1 protein expression for patients with advanced NSCLC: any differences based on histology? Clin. Lung Cancer. 2023;24(5):401–406. doi: 10.1016/j.cllc.2023.03.014. [DOI] [PubMed] [Google Scholar]
- 2.Hong D.S., Fakih M.G., Strickler J.H., Desai J., Durm G.A., Shapiro G.I., Falchook G.S., Price T.J., Sacher A., Denlinger C.S., et al. KRAS(G12C) inhibition with sotorasib in advanced solid tumors. N. Engl. J. Med. 2020;383(13):1207–1217. doi: 10.1056/NEJMoa1917239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Canon J., Rex K., Saiki A.Y., Mohr C., Cooke K., Bagal D., Gaida K., Holt T., Knutson C.G., Koppada N., et al. The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature. 2019;575(7781):217–223. doi: 10.1038/s41586-019-1694-1. [DOI] [PubMed] [Google Scholar]
- 4.U.S. Food and Drug Administration Drugs@FDA [database on the internet] Sotorasib USPI. 2023 1-9. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/214665s000lbl.pdf December 11, 2023. [Google Scholar]
- 5.Nakajima E.C., Drezner N., Li X., Mishra-Kalyani P.S., Liu Y., Zhao H., Bi Y., Liu J., Rahman A., Wearne E., et al. FDA approval summary: sotorasib for KRAS G12C-mutated metastatic NSCLC. Clin. Cancer Res. 2022;28(8):1482–1486. doi: 10.1158/1078-0432.CCR-21-3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Langen A.J., Johnson M.L., Mazieres J., Dingemans A.C., Mountzios G., Pless M., Wolf J., Schuler M., Lena H., Skoulidis F., et al. Sotorasib versus docetaxel for previously treated non-small-cell lung cancer with KRAS(G12C) mutation: a randomised, open-label, phase 3 trial. Lancet. 2023;401(10378):733–746. doi: 10.1016/S0140-6736(23)00221-0. [DOI] [PubMed] [Google Scholar]
- 7.Strickler J.H., Satake H., George T.J., Yaeger R., Hollebecque A., Garrido-Laguna I., Schuler M., Burns T.F., Coveler A.L., Falchook G.S., et al. Sotorasib in KRAS p.G12C-mutated advanced pancreatic cancer. N. Engl. J. Med. 2023;388(1):33–43. doi: 10.1056/NEJMoa2208470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fakih M.G., Kopetz S., Kuboki Y., Kim T.W., Munster P.N., Krauss J.C., Falchook G.S., Han S.W., Heinemann V., Muro K., et al. Sotorasib for previously treated colorectal cancers with KRAS(G12C) mutation (CodeBreaK100): a prespecified analysis of a single-arm, phase 2 trial. Lancet Oncol. 2022;23(1):115–124. doi: 10.1016/S1470-2045(21)00605-7. [DOI] [PubMed] [Google Scholar]
- 9.Fakih M.G., Salvatore L., Esaki T., Modest D.P., Lopez-Bravo D.P., Taieb J., Karamouzis M.V., Ruiz-Garcia E., Kim T.W., Kuboki Y., et al. Sotorasib plus panitumumab in refractory colorectal cancer with mutated KRAS G12C. N. Engl. J. Med. 2023;389(23):2125–2139. doi: 10.1056/NEJMoa2308795. [DOI] [PubMed] [Google Scholar]
- 10.Skoulidis F., Li B.T., Dy G.K., Price T.J., Falchook G.S., Wolf J., Italiano A., Schuler M., Borghaei H., Barlesi F., et al. Sotorasib for lung cancers with KRAS p.G12C mutation. N. Engl. J. Med. 2021;384(25):2371–2381. doi: 10.1056/NEJMoa2103695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dy G.K., Govindan R., Velcheti V., Falchook G.S., Italiano A., Wolf J., Sacher A.G., Takahashi T., Ramalingam S.S., Dooms C., et al. Long-term outcomes and molecular correlates of sotorasib efficacy in patients with pretreated KRAS G12C-mutated non-small-cell lung cancer: 2-year analysis of CodeBreaK 100. J. Clin. Oncol. 2023;41(18):3311–3317. doi: 10.1200/JCO.22.02524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang S.S., Lee A., Nagasaka M. CodeBreak 200: sotorasib has not broken the KRAS(G12C) enigma code. Lung Cancer. 2023;14:27–30. doi: 10.2147/LCTT.S403461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shu Y., Ding Y., Liu L., Zhang Q. Cardiac adverse events associated with quetiapine: disproportionality analysis of FDA adverse event reporting system. CNS Neurosci. Ther. 2023;29(9):2705–2716. doi: 10.1111/cns.14215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shu Y., Wang L., Ding Y., Zhang Q. Disproportionality analysis of abemaciclib in the FDA adverse event reporting system: a real-world post-marketing pharmacovigilance assessment. Drug Saf. 2023;46(9):881–895. doi: 10.1007/s40264-023-01334-z. [DOI] [PubMed] [Google Scholar]
- 15.Setyawan J., Azimi N., Strand V., Yarur A., Fridman M. Reporting of thromboembolic events with JAK inhibitors: analysis of the FAERS database 2010-2019. Drug Saf. 2021;44(8):889–897. doi: 10.1007/s40264-021-01082-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu Y., Gong J., Zhang L., Li X., Li X., Zhao B., Hai X. Colitis following the use of immune checkpoint inhibitors: a real-world analysis of spontaneous reports submitted to the FDA adverse event reporting system. Int. Immunopharm. 2020;84 doi: 10.1016/j.intimp.2020.106601. [DOI] [PubMed] [Google Scholar]
- 17.Yan Y.D., Zhao Y., Zhang C., Fu J., Su Y.J., Cui X.L., Ma E.L., Liu B.L., Gu Z.C., Lin H.W. Toxicity spectrum of immunotherapy in advanced lung cancer: a safety analysis from clinical trials and a pharmacovigilance system. EClinicalMedicine. 2022;50 doi: 10.1016/j.eclinm.2022.101535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shu Y., Ding Y., He X., Liu Y., Wu P., Zhang Q. Hematological toxicities in PARP inhibitors: a real-world study using FDA adverse event reporting system (FAERS) database. Cancer Med. 2023;12(3):3365–3375. doi: 10.1002/cam4.5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mazhar F., Battini V., Gringeri M., Pozzi M., Mosini G., Marran A.M.N., Akram S., van Manen R.P., Radice S., Clementi E., et al. The impact of anti-TNFα agents on weight-related changes: new insights from a real-world pharmacovigilance study using the FDA adverse event reporting system (FAERS) database. Expet Opin. Biol. Ther. 2021;21(9):1281–1290. doi: 10.1080/14712598.2021.1948529. [DOI] [PubMed] [Google Scholar]
- 20.Cornelius V.R., Sauzet O., Evans S.J. A signal detection method to detect adverse drug reactions using a parametric time-to-event model in simulated cohort data. Drug Saf. 2012;35(7):599–610. doi: 10.2165/11599740-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 21.Leiter A., Veluswamy R.R., Wisnivesky J.P. The global burden of lung cancer: current status and future trends. Nat. Rev. Clin. Oncol. 2023;20(9):624–639. doi: 10.1038/s41571-023-00798-3. [DOI] [PubMed] [Google Scholar]
- 22.Chour A., Denis J., Mascaux C., Zysman M., Bigay-Game L., Swalduz A., Gounant V., Cortot A., Darrason M., Fallet V., et al. Brief report: severe sotorasib-related hepatotoxicity and non-liver adverse events associated with sequential anti-programmed cell death (Ligand)1 and sotorasib therapy in KRAS(G12C)-Mutant lung cancer. J. Thorac. Oncol. 2023;18(10):1408–1415. doi: 10.1016/j.jtho.2023.05.013. [DOI] [PubMed] [Google Scholar]
- 23.Luo G., Huang L., Zhang Z. The molecular mechanisms of acetaminophen-induced hepatotoxicity and its potential therapeutic targets. Exp. Biol. Med. 2023;248(5):412–424. doi: 10.1177/15353702221147563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Motafeghi F., Mortazavi P., Ghassemi-Barghi N., Zahedi M., Shokrzadeh M. Dexamethasone as an anti-cancer or hepatotoxic. Toxicol. Mech. Methods. 2023;33(2):161–171. doi: 10.1080/15376516.2022.2105183. [DOI] [PubMed] [Google Scholar]
- 25.Zhang H., Wu J., Zhang Z., Qian H., Wang Y., Yang M., Cheng Y., Tang S. Association of atorvastatin with the risk of hepatotoxicity: a pilot prescription sequence symmetry analysis. Therapeut. Clin. Risk Manag. 2019;15:803–810. doi: 10.2147/TCRM.S204860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manov I., Bashenko Y., Hirsh M., Iancu T.C. Involvement of the multidrug resistance P-glycoprotein in acetaminophen-induced toxicity in hepatoma-derived HepG2 and Hep3B cells. Basic Clin. Pharmacol. Toxicol. 2006;99(3):213–224. doi: 10.1111/j.1742-7843.2006.pto_443.x. [DOI] [PubMed] [Google Scholar]
- 27.Bourdin V., Bigot W., Vanjak A., Burlacu R., Lopes A., Champion K., Depond A., Amador-Borrero B., Sene D., Comarmond C., et al. Drug-drug interactions involving dexamethasone in clinical practice: myth or reality? J. Clin. Med. 2023;12(22) doi: 10.3390/jcm12227120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boyd R.A., Stern R.H., Stewart B.H., Wu X., Reyner E.L., Zegarac E.A., Randinitis E.J., Whitfield L. Atorvastatin coadministration may increase digoxin concentrations by inhibition of intestinal P-glycoprotein-mediated secretion. J. Clin. Pharmacol. 2000;40(1):91–98. doi: 10.1177/00912700022008612. [DOI] [PubMed] [Google Scholar]
- 29.Sabari J.K., Velcheti V., Shimizu K., Strickland M.R., Heist R.S., Singh M., Nayyar N., Giobbie-Hurder A., Digumarthy S.R., Gainor J.F., et al. Activity of adagrasib (MRTX849) in brain metastases: preclinical models and clinical data from patients with KRASG12C-mutant non-small cell lung cancer. Clin. Cancer Res. 2022;28(15):3318–3328. doi: 10.1158/1078-0432.CCR-22-0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vassella E., Kashani E., Zens P., Kündig A., Fung C., Scherz A., Herrmann E., Ermis E., Schmid R.A., Berezowska S. Mutational profiles of primary pulmonary adenocarcinoma and paired brain metastases disclose the importance of KRAS mutations. Eur. J. Cancer. 2021;159:227–236. doi: 10.1016/j.ejca.2021.10.006. [DOI] [PubMed] [Google Scholar]
- 31.Cutroneo P.M., Sartori D., Tuccori M., Crisafulli S., Battini V., Carnovale C., Rafaniello C., Capuano A., Poluzzi E., Moretti U., et al. Conducting and interpreting disproportionality analyses derived from spontaneous reporting systems. Frontiers in Drug Safety and Regulation. 2024;3 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data included in article/supplementary/referenced in article.