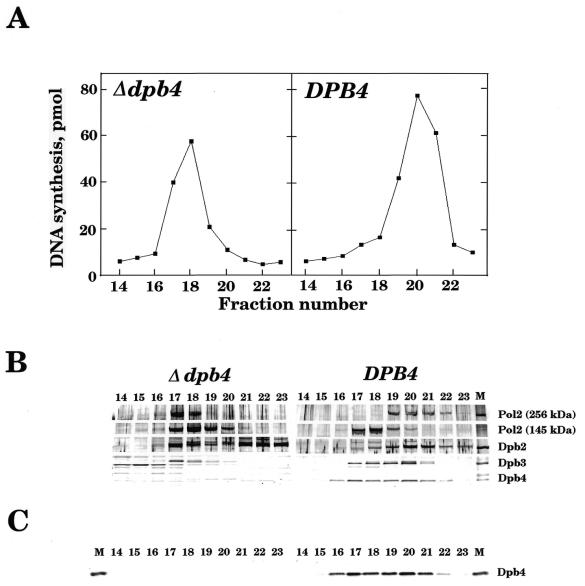
Figure 1.
Partial purification of Polɛ complex from wild-type and Δdpb4 mutant yeast cells. Yeast cell extracts were prepared, applied to an S–Sepharose column and the proteins retained on the column were eluted with buffer A containing 0.5 M NaCl as described previously (11). The eluate was dialysed against buffer A, 0.1 M NaCl, applied to a Mono Q column equilibrated with the same buffer, and Polɛ was eluted with a linear gradient from 0.1 to 0.5 M NaCl in buffer A (11). Assays for the activity of Polɛ were carried out using oligo(dT)10/poly(dA)>500 (1:50) as a template-primer (A) and the fractions were analysed by SDS–PAGE and western blotting (6). Filters were probed with rabbit antibodies against Polɛ complex (21) (B) and Dpb4p (C). The purified Polɛ complex (11) (20 ng) was also used as a control (M) in (B) and (C). The numbers shown in the Figure are the fraction numbers after Mono Q column chromatography.