FIGURE 2.
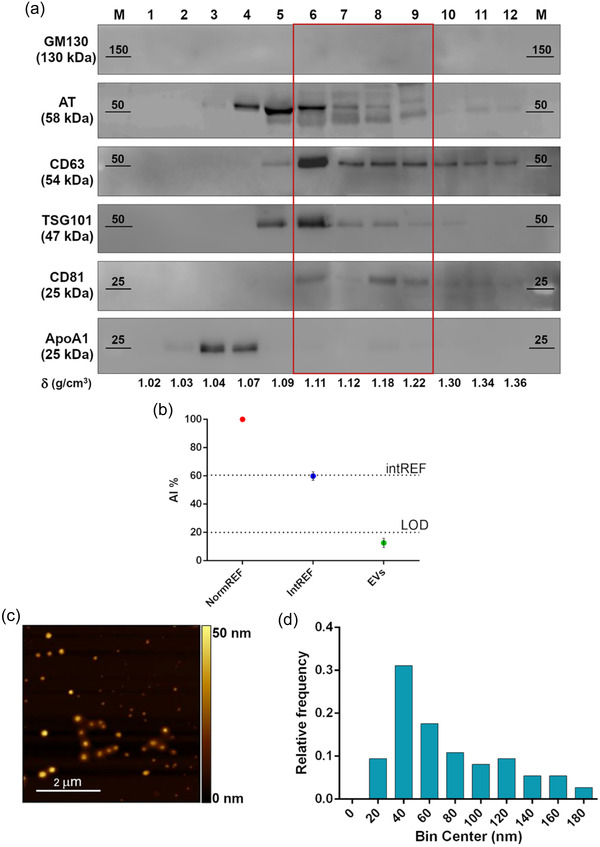
Comprehensive characterization of healthy plasma pool derived EVs. (a) Western blot analysis after SDS‐PAGE of sucrose gradient fractions in reducing conditions. The distribution of EV (CD63, TSG101, CD81), Golgi (GM130, negative control), and lipoprotein (ApoA1, contaminant) markers is shown, together with AT. Fractions from 6 to 9, highlighted by the red rectangle, feature the absence of contaminants and the co‐localization of AT and EV markers. (b) CONAN assay performed on the EV samples indicate that the isolation method used allows to obtain EV preparation with negligible amounts of soluble protein contaminants (soluble AT included). AI% value of a representative EV sample (green spot) is showed, together with assay internal controls (red and blue dots). (c) Representative AFM image of the particles in fractions six to nine, displaying intact and round shape objects, and a size comparable to the one of EV dried on a surface. (d) Size distribution of the particles in fractions six to nine, imaged through AFM. Particle diameter was extrapolated from 200 objects
