FIGURE 2.
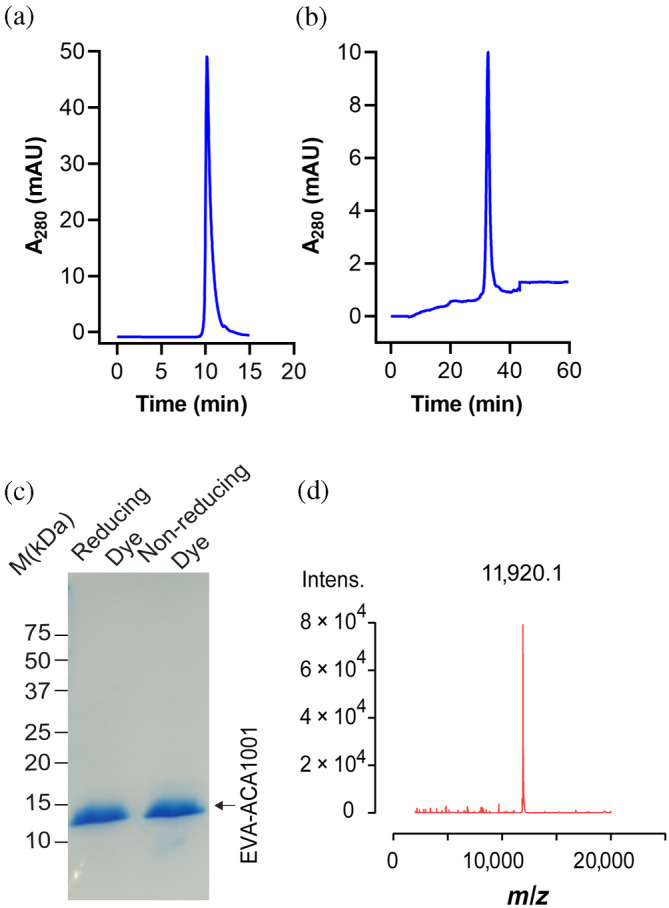
Quality control analysis of purified EVA‐ACA1001. (a) Analytical size‐exclusion chromatography (column: Yarra TM 3 μm SEC‐2000 Phenomenex; eluent: 0.1 M NaH2PO4 pH 7.5; and flow rate: 0.35 mL/min) (b) analytical reversed‐phase high‐performance liquid chromatography (column: Protein C4 214TP5315 GRACE VYDAC; eluent: a mobile phase of 0.1% trifluoroacetic acid in water (Solvent A) and 0.1% trifluoroacetic acid in acetonitrile (Solvent B) with a linear gradient of 0–60% B over 60 min; and flow rate: 0.35 μL/min). (c) Coomassie blue‐stained SDS‐polyacrylamide gel showing purified EVA‐ACA1001 in reducing and nonreducing conditions; protein molecular weight markers are shown as black lines and labeled in kDa; lane 1, EVA‐ACA1001 in reducing conditions; and lane 2, EVA‐ACA1001 in nonreducing conditions. (d) Analysis of EVA‐ACA1001 by mass spectrometry. Purified EVA‐ACA1001 was analyzed by liquid chromatography‐electrospray ionization mass spectrometry (LC MS‐ESI). The expected mass of EVA‐ACA1001 with five disulfide bonds is 11,921.14 Da.
