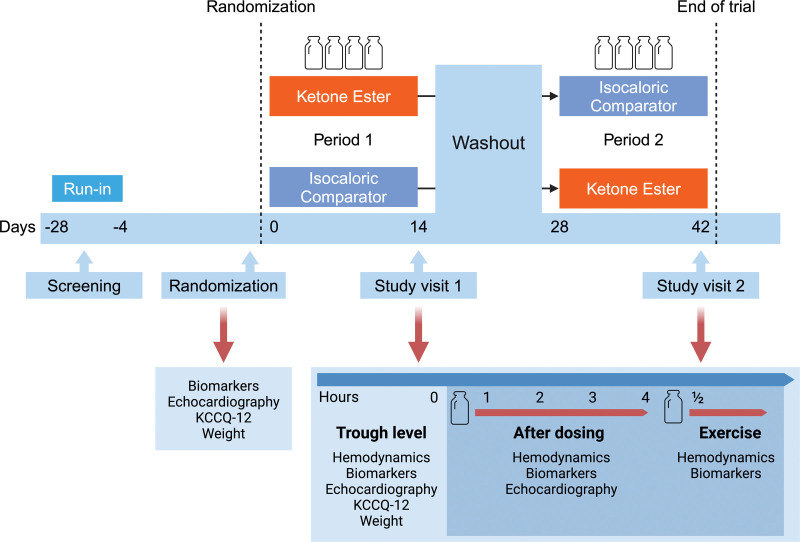
Figure 1.
Study design. A randomized, double-blind, isocaloric comparator–controlled cross-over trial in 24 stable patients with heart failure with reduced ejection fraction. Participants underwent a 14-day treatment with oral ketone ester and an isocaloric comparator, after which they were evaluated with right-sided heart catheterization, echocardiography, blood sampling, Kansas City Cardiomyopathy Questionnaire (KCCQ-12), and exercise hemodynamics. Study periods were separated by a 14-day washout period. A 1-day run-in period of each intervention after screening was planned to ensure tolerability before enrollment.