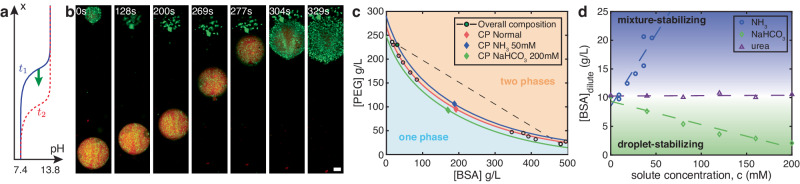
Fig. 3. Droplet motility driven by perturbations of phase equilibria.
a Schematic of the sharp pH front propagating from top to bottom in (b). b Image sequence of passive condensates moving and dissolving in response to the sharp pH front. Scale bar, 10 μm (see also Movie S4, PEGDA 12k coating). c Phase diagram of the PEG-BSA system. In the absence of solute, circles show the measured binodal19 and a red diamond shows the critical point. We prepare our droplets at the overall composition indicated by the green circle, and the system phase-separates along the associated tie line (dashed line and white circles). The background colors and colored curves are guides to the eye. Ezymatic products shift the critical point (blue 50 mM NH3 (aq) and green 200 mM NaHCO3 diamonds). d Shifts of BSA concentration in the dilute-phase as solutes NH3 (aq), NaHCO3 and urea are added at fixed overall BSA and PEG concentrations. Dashed lines are linear fits and shaded areas are guides to the eye. Source data for (c, d) are provided as a Source Data file.