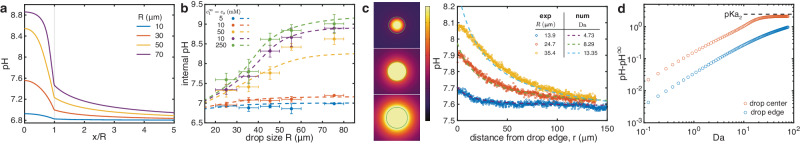
Fig. 6. Reaction-diffusion simulations.
a pH profiles as a function of the dimensionless distance x/R as the drop radius R increases (pH∞ = 6.8, ηdense = 0.8 Pa s, mM, μM, kcat = 4400 1/s). b Internal pH as a function of the drop radius R for various urea concentrations cs. Data extracted from ref. 19, circles are mean values and vertical error bar the SD of the internal pH measured in 5 independent droplets. Horizontal error bars represent the edges of the bin used to bin the data in ref. 19. Dashed lines are simulations (same parameters as a, except for cs that is varied). c Numerical prediction for the pH around the reacting droplets shown in Fig. 1f (μM, kcat = 352 1/s, pH∞ = {7.59, 7.59, 7.54} and ηdense = {0.8, 0.3, 0.2} Pa s). Images of the numerical simulations are shown on the left (the green circles indicates the drop edge), and pH profiles outside of the drop extracted from the experiments and simulations are shown on the right. d Increase in pH due to the activity pH − pH∞ as a function of the Damköhler numbers Da in the drop center and at the edge (same parameters as a). The black dashed line is pH = pKa2. Source data for (a–d) are provided as a Source Data file.