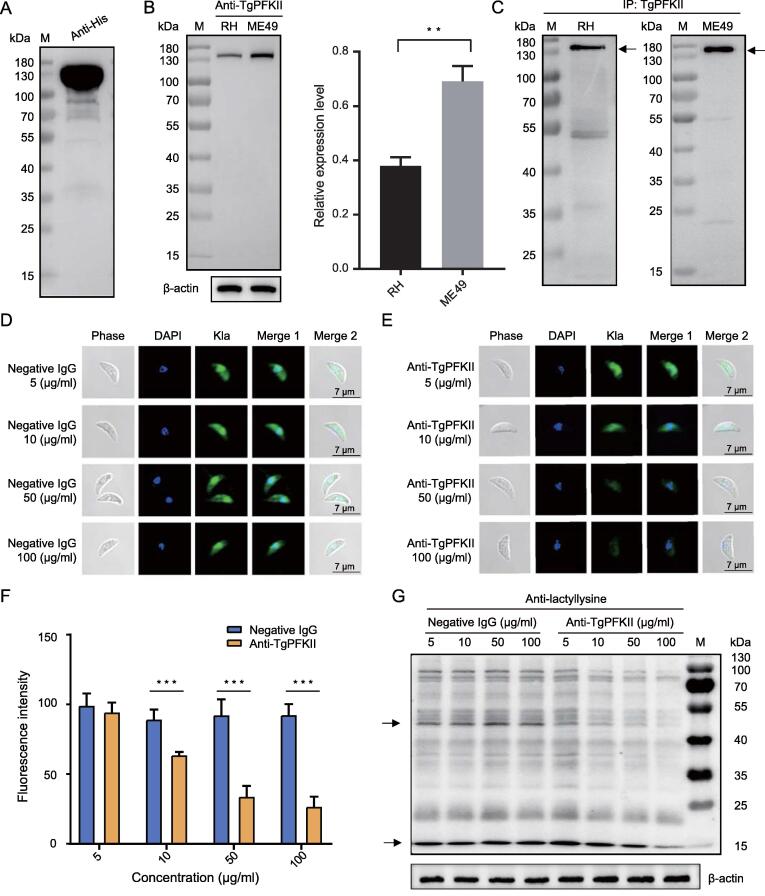
Figure 5.
Anti-TgPFKII antibodies reduced protein lactylation
A. The purified His-tagged TgPFKII was verified by Western blotting using the anti-His antibody. B. Western blotting analysis of TgPFKII expression levels (131 kDa) in T. gondii RH and ME49 using the anti-TgPFKII antibody. β-actin was used for normalization. The data are presented as mean ± standard deviation of three independent experiments (*, P < 0.05; **, P < 0.01; Student’s t-test). C. The soluble proteins derived from T. gondii RH and ME49 were immunoprecipitated with an anti-TgPFKII antibody, and the TgPFKII protein (arrow-headed) precipitated was detected by the anti-TgPFKII antibody. D. No effect on protein lactylation (green) with different concentrations of negative IgG (mouse IgG) analyzed by IFA. E. The anti-TgPFKII antibodies inhibited protein lactylation (green) in a concentration-dependent manner. F. Immunofluorescence intensity was statistically analyzed for the groups treated with the anti-TgPFKII antibody and the negative control antibody, respectively. Statistical significance analysis was performed using Student’s t test. All data are shown as mean ± standard deviation (n > 10). ***, P < 0.001. G. Western blotting assay was used to test the effect of different concentrations of anti-TgPFKII antibodies on the Kla level of T. gondii RH. β-actin was used for normalization. IP, immunoprecipitation; PFKII, phosphofructokinase II.