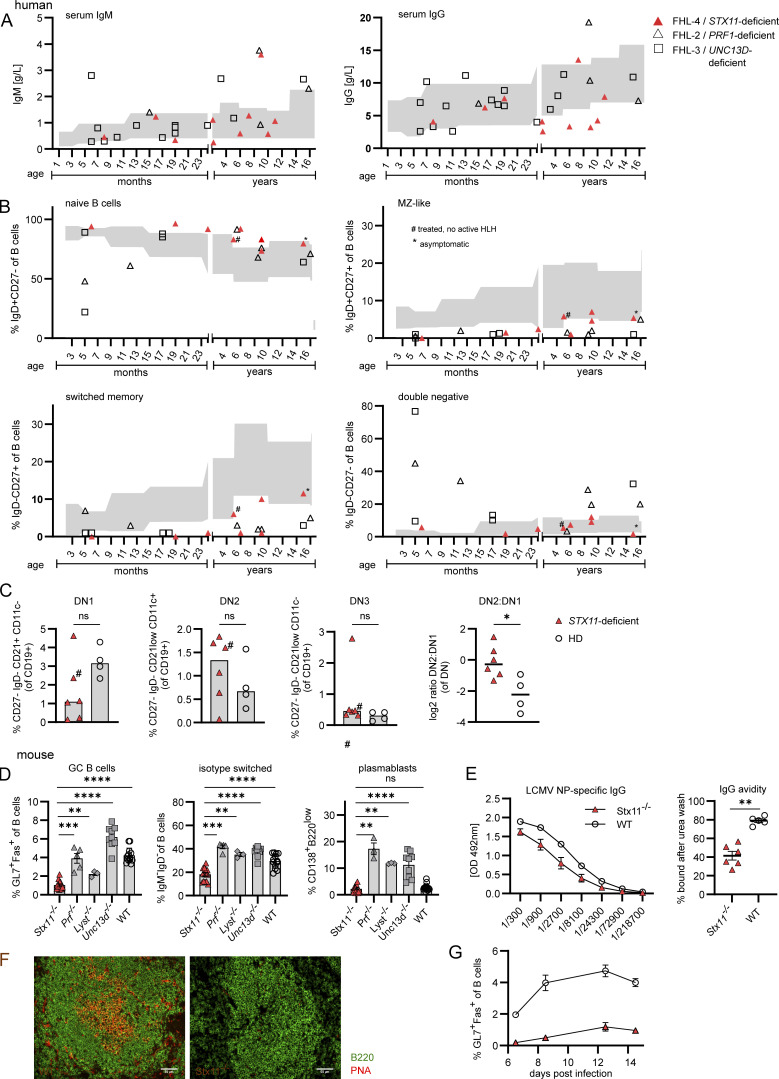
Figure 1.
Hypogammaglobulinemia and impaired GC formation in FHL-4 patients and Stx11−/− mice. Human: (A and B) Gray area: age-related reference values (Oster, 2015; Piątosa et al., 2010); # treated; * asymptomatic. (A) Sera from 4 FHL-2 (PRF1-deficiency), 16 FHL-3 (UNC13D-deficiency), and 10 FHL-4 (STX11-deficiency) patients with acute HLH, (≥5 mo of age). i.v. Ig–substituted patients were excluded. (B) B cell subpopulations in human samples shown as % of total B cells (six FHL-2, five FHL-3, and eight FHL-4 patients): IgD+CD27− (naive), IgD+CD27+ (MZ-like), IgD−CD27+ (switched memory), and IgD−CD27− (DN). (C) DN subpopulations of frozen PBMC samples of six FHL-4 patients and four healthy donors (HD). DN1: differentiation via GCs, DN2: extrafollicular differentiation. Mouse: (D) Frequency of isotype-switched B cells, GC B cells (Fas+GL7+), isotype-switched B cells (IgM−IgD− of CD19+B220+), and plasmablasts (CD138+B220low) in spleens of Stx11−/− (n = 15, five independent experiments), Prf1−/−(n = 5, two independent experiments), Unc13d−/− (n = 9, three independent experiments), and Lyst−/− (n = 3, one experiment) and WT (C57BL/6N n = 4, Stx11+/+ n = 9, five independent experiments) mice d12–14 p.i. with 200 PFU LCMV-WE i.v. (E) LCMV-NP–specific IgG in sera of Stx11−/− and WT mice on d28 p.i. analyzed by ELISA (WT: C57BL/6N n = 1 and Stx11+/+ n = 4, Stx11−/− n = 7, one experiment). IgG avidity measurements. (WT: C57BL/6N n = 1 and Stx11+/+ n = 4, Stx11−/− n = 7, one representative experiment). (F) Representative spleen sections stained for B220 (green) and PNA (red) (n = 4 independent experiments). Scale bar 50 µm. (G) Stx11−/− (n = 6–8) and WT littermates (Stx11+/+) (n = 4–10) were infected with 200 PFU LCMV i.v. d14 p.i.: GC B cells and their % over time (n = 2 independent experiments). (A–G) Data are shown as mean and SEMs. Statistical analysis was performed using Mann–Whitney U test; *P < 0.05, **P < 0.01, *** P < 0.001, **** < 0.0001, ns indicates not significant.