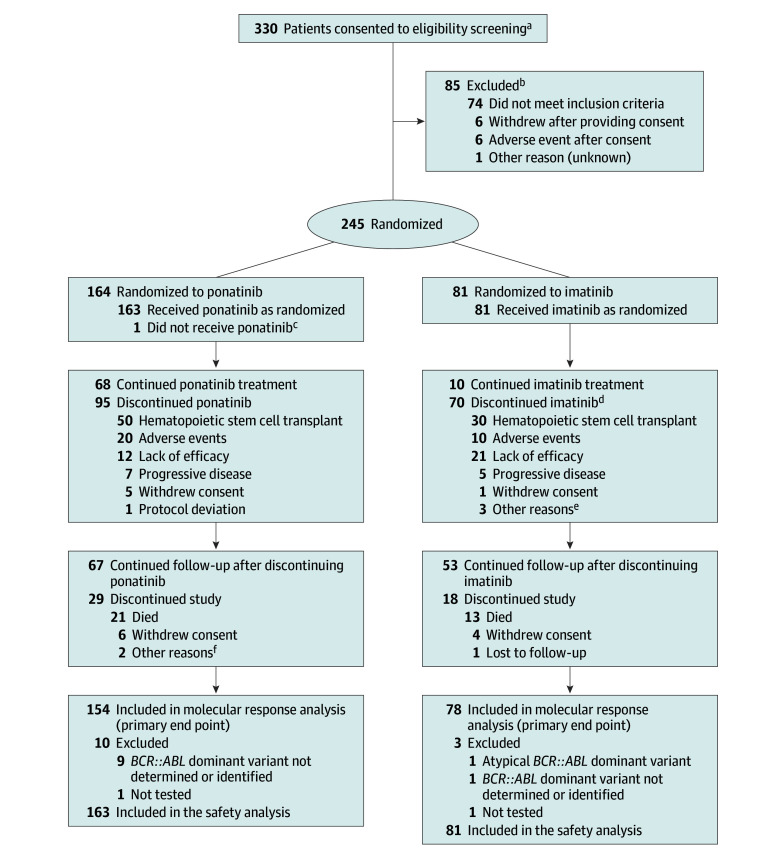
Figure 1. Participant Flow in the PhALLCON Trial.
aThere were 332 screening assessments of 330 patients; 2 patients were rescreened after not meeting eligibility initially. One patient approached for eligibility screening did not consent.
bThe 2 patients rescreened as mentioned in footnote a are included in reasons for exclusion but are not counted in the total number of patients excluded (n = 85).
cThis patient died of COVID-19 after randomization and before receiving any dose of ponatinib.
dReasons for discontinuation derived from electronic case report forms completed by study site personnel. One patient randomized to imatinib was discontinued due to a fatal adverse event; the reason for discontinuation was not reported.
eOther reasons included loss of minimum residual disease negativity and investigator decision to start another treatment (n = 1), loss of cytogenic response (n = 1), and patient not agreeing to study procedure and polymerase chain reaction not at an ideal level (n = 1).
fOther reasons included nonadherence to treatment visits and no longer meeting inclusion criteria (n = 1) and a report of “completed” (additional information not available; n = 1).