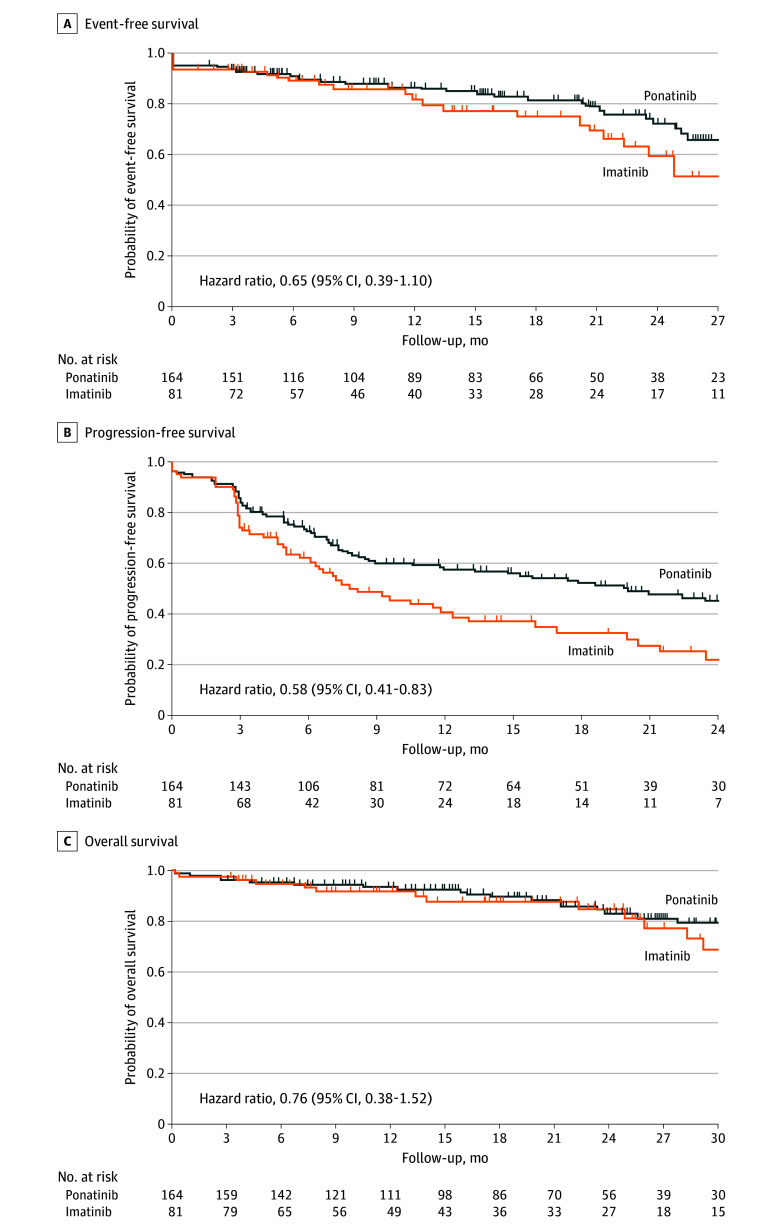
Figure 2. Kaplan-Meier Estimates of Survival Outcomes in the Intention-to-Treat Population.
A, Median event-free survival was not estimable (NE) (95% CI, NE-NE) with ponatinib and was 29.0 months (95% CI, 22.3 months–NE) with imatinib, with median follow-up of 17.3 (IQR, 7.3-26.3) months and 15.9 (IQR, 7.0-28.9) months, respectively. Event-free survival occurred in 34 patients in the ponatinib group and 24 patients in the imatinib group. B, Median progression-free survival was 20.0 months (95% CI, 11.8 months–NE) with ponatinib and 7.9 (95% CI, 6.2-12.4) months with imatinib, with median follow-up of 20.7 (IQR, 12.5-29.5) months and 19.2 (IQR, 10.8-35.0) months, respectively. Progression-free survival occurred in 77 patients in the ponatinib group and 54 patients in the imatinib group. C, Median overall survival was NE (95% CI, NE-NE) in the ponatinib group and NE (95% CI, 29.0 months–NE) in the imatinib group, with median follow-up of 20.4 (IQR, 10.4-29.5) months and 18.1 (IQR, 9.8-30.5) months, respectively. Overall survival occurred in 21 patients in the ponatinib group and 13 patients in the imatinib group. Patients who received hematopoietic stem cell transplant were censored (event-free survival: 47/164 [28.7%] for ponatinib and 28/81 [34.6%] for imatinib; progression-free survival: 25/164 [15.2%] for ponatinib and 15/81 [18.5%] for imatinib; overall survival: 48/164 [29.3%] for ponatinib and 34/81 [42.0%] for imatinib).