Abstract
Different stress models are employed to enhance our understanding of the underlying mechanisms and explore potential interventions. However, the utility of these models remains a critical concern, as their validities may be limited by the complexity of stress processes. Literature review revealed that both mental and physical stress models possess reasonable construct and criterion validities, respectively reflected in psychometrically assessed stress ratings and in activation of the sympathoadrenal system and the hypothalamic-pituitary-adrenal axis. The findings are less robust, though, in the pharmacological perturbations’ domain, including such agents as adenosine or dobutamine. Likewise, stress models’ convergent- and discriminant validity vary depending on the stressors’ nature. Stress models share similarities, but also have important differences regarding their validities. Specific traits defined by the nature of the stressor stimulus should be taken into consideration when selecting stress models. Doing so can personalize prevention and treatment of stress-related antecedents, its acute processing, and chronic sequelae. Further work is warranted to refine stress models’ validity and customize them so they commensurate diverse populations and circumstances.
Keywords: allostasis, arginine-vasopressin, cortisol, homeostasis, immune, norepinephrine, psychodiagnostic, psychometric, renin-angiotensin-aldosterone
1. Introduction
If You Canť Measure It, You Canť Improve It.
Peter Ferdinand Drucker
The impact of disastrously stressful experiences on well-being is overwhelming and is evident in the atrocities at the southern border of Israel (Rees and Moussa, 2023), the war in Ukraine (Mottola et al., 2023), mass shootings (Lowe and Galea, 2017), the coronavirus pandemic (Whiteman et al., 2023), and economic inflation and downturns (Louie et al., 2023). Maladaptive responses to stress are associated with serious illnesses, including, addictions, allergies, anxiety disorders, autoimmune disorders, chronic fatigue syndrome, hypertension, major depression, metabolic syndrome, pain syndromes, post-traumatic stress disorder (PTSD), psychosis, and infections (Chrousos and Gold, 1992; Elman et al., 1998; Elman and Borsook, 2019; Elman et al., 2006; Gold and Chrousos, 2002; Swaab et al., 2005; Tsigos and Chrousos, 1994). To compound the affront, stress is also an inevitable aspect of day-to-day life in the form of aggravating or unpleasant experiences (Elman et al., 2010).
Acute stress is essential for successful coping and survival by improving performance and avoidance of harm via the "fight-or-flight" type of coping style (Cannon 1915). Normally, the well-orchestrated homeostatic machinery is infallible in the maintenance of equilibrium across body systems and for the stabilization of emotional states and motivational drives during routine stressors (Elman and Borsook, 2016). However, enormous, recurrent, or continuous stresses induce robust and sustained systems' strain that override homeostatic feedback control to generate allostatic adaptations (Elman and Borsook, 2019; Elman et al., 2011). Being unchecked by physiological negative feedback mechanisms, these adaptations lead to an autonomous, self-sustaining feedforward loop, whereby prior exposure to one type of stress increases the subsequent response to itself and to a different type of stress (Elman et al., 2013; Juruena, 2014; Rao and Androulakis, 2019). And so, understanding the mechanisms by which stress affects the mind and body is essential for the development of effective interventions to mitigate stress’ negative impacts.
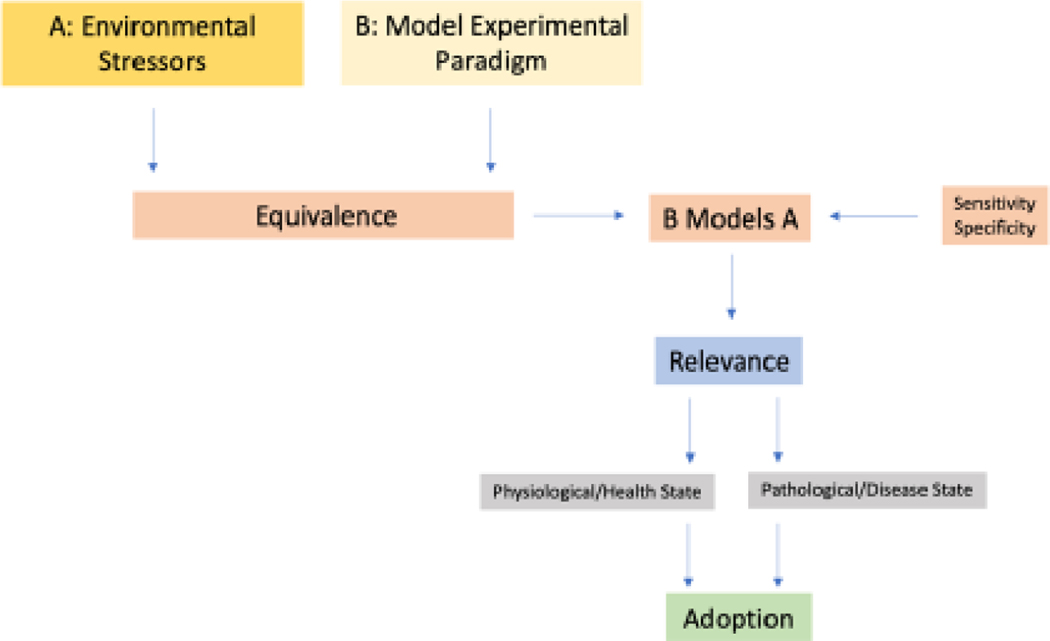
The present review addresses stress with its pathophysiological sequalae by considering the validity of the prevailing stress models i.e., systematic frameworks incorporating stressors, measurable variables, and test paradigms to simulate and understand stress responses (Crosswell and Lockwood, 2020). To that end, we emphasize dimensional and interactive conceptualization of stress, using conventional challenges perturbing the regulatory systems. Instead of strictly adhering to a single model, our approach offers an optimal means of comprehensively understanding the biopsychosocial aspects of stress. Figure 1 depicts a schematic flowchart that outlines the process of identifying and establishing potential evaluation models. This process is akin to the one employed in adopting biomarkers for drug discovery, as described by Borsook et al., 2011. The idea is that specific experimental stress models should adhere to specific criteria concerning their validity, reproducibility, and biological significance.
Figure 1:
What do we measure in Experimental Models of Stress? Stressors are ubiquitous across environmental, social, individual, and societal processes. Models of stress conducted in the laboratory need to capture acute and chronic stressors across health and disease and to understand the implications of such stressors on disease initiation, progression, and resistance to treatment.
At the outset, we delve into the challenges of defining and evaluating stress, discuss various stress conceptualizations and differentiate between non-specific bodily responses and specific organ reactions to stress considering individual and environmental influences on stress symptoms. The interaction between mental and physical aspects of stress is also detailed, exemplifying how stress outcomes are unique for each person. Then various validity types of stress models (Table 1) are addressed including the value of objective assessments alongside self-reports for validity assessments. The body's response to stress involves several regulatory systems, including the sympathoadrenal-, hypothalamic-pituitary-adrenal (HPA)-, renin-angiotensin-aldosterone- (RAAS), arginine-vasopressin- (AVP), and cytokine immune systems (Bali and Jaggi, 2013; Jezova et al., 1995; Tian et al., 2014). Because the ‘fight or flight’ hormones and neurochemicals (Goldstein, 2010) released by the sympathoadrenal system and HPA axis may be construed as a gold standard stress criterion (Schlotz, 2013), an a priori emphasis is placed on the discussion of their role in regulating emotional adaptation, blood pressure, water balance, immune defense, and inflammation in response to internal and external challenges. Next, we discuss how the sympathoadrenal system and HPA axis work together with other systems to restore and maintain homeostasis (Chrousos, 2009) and how various stress models’ validity converge and discriminate, depending on the nature of the stressor stimulus, including its duration (acute vs. chronic) and type (natural vs. man-made). Finally, we provide a summary and conclusions.
Table 1:
Stress-related types of validity
| Validity | Definition | Applicability to Stress |
|---|---|---|
| Construct | Measure of compatibility between the employed model and the concept per se. | Complementary psycho-diagnostic and -metric tools as measures of the stress construct. |
| Criterion | Compatibility of the employed model with the gold standard characteristic of construct it purports to represent. |
Activation of the sympathoadrenal system and HPA axis are commonly accepted characteristics of stress responses. |
| Convergent | Compatibility with other measures of the same construct. | Stress commonly co-occurs with depression, anxiety, tension, confusion, tiredness, anger, disappointment, etc. (Elman et al., 2010; Williamson et al., 2021) |
| Discriminatory | Unique aspects of the applied model. | Assessment whether different stress models, which might be designed to measure various aspects or types of stress, are indeed distinct from one another and not overlapping in terms of the psychological constructs they aim to measure. |
The intentional limitation of the background information and literature review to issues directly relevant to validity of stress models has resulted in some degree of oversimplification. While we recognize the importance of a comprehensive review of stress physiology engaging many other hormones e.g., neuropeptide Y (Reichmann and Holzer, 2016), cholecystokinin (Siegel et al., 1987), or substance P (Iftikhar et al., 2020), the discussion of which would go well beyond the scope of this review. Moreover, as this review draws heavily upon animal models (e.g., social defeat) that are valuable tools for understanding specific aspects of stress biology their findings should be interpreted cautiously (Bracken, 2009). It is important to highlight many uncertainties inherent in extrapolating from animal data to human conditions (Elman et al., 2006) such as a higher level of cognitive, motivational, and emotional complexity compared to animals e.g., intricate social and interpersonal interactions, wide ranging behavioral outcomes’ repertoire, cultural nuances, and societal expectations. That said, the animal models provide a platform for testing the efficacy of behavioral and pharmacological interventions with positive outcomes suggesting potential avenues for human therapeutic development (Ferreira et al., 2019; Singh and Seed, 2021).
2. Search Terms and Methodology
English language literature search of normal stress mechanisms and their potential impairments in patients with chronic stress conditions was undertaken using PubMed, Google Scholar, and Westlaw from inception until October 2023. The search terms included “allostasis,” “adenosine,” “AVP,” “cytokine,” “dobutamine,” “glucoprivation,” “heat,” “homeostasis,” “HPA axis,” “hypovolemia,” immune,” “infection,” “interleukin,” “Maastricht acute stress test (MAST),” “(neuro)chemical,” “(neuro)endocrine,” “pain,” “post-traumatic stress disorder (PTSD),” “RAAS,” “sleep deprivation,” “social defeat,” “stress,” “Stroop test,” “sympathoadrenal,” “Trier social stress test (TSST),” and “yohimbine.” Data on stress mechanisms and stress-related morbidity were also drawn from seminal reviews of these topics (Charney, 2004; de Filippi, 1991; de Kloet et al., 2019; de Kloet et al., 2005; Goldstein, 2021; Koob and Schulkin, 2019; McEwen, 2000, 2020; O'Connor et al., 2021; Rodrigues et al., 2009; Tsigos et al., 2000). Additional strategies included manual searches for relevant articles from the selected papers’ reference lists as well as utilization of PubMed’s related articles’ function.
3. Neuroanatomical and Neuroendocrine Stress Determinants
The investigation of stress is often hindered by a fundamental question: How to define, evaluate, or operationalize "stress"? This concept has been given multiple definitions across various disciplines and standpoints e.g., cognitive, emotional, neurobiological, pathophysiological, and molecular. Yet, even among these conceptualizations, widely accepted definitions exhibit circularity, like the concept of “forced exposure to events or conditions that are typically avoided,” which essentially defines stress as stressful experiences (Epstein et al., 2006; Piazza and Le Moal, 1998). Nonetheless, the neuroanatomical and neuroendocrine stress determinants appear to be rigorously established and generally agreed-upon, which could shed more light on the mental and physical stress’ dimensions, its diverse presentations, and potential interventions.
Neural stress domain comprises the sensory apparatus (Gregoric, 2007), viz., organs such as the skin, eyes, ears, nose and tongue (Marzvanyan and Alhawaj, 2023), as well as the associated neural networks that process sensory information and transmit it via afferent neurons to the thalamus. The thalamus relays the sensory input to dedicated visual-, auditory-, and somatosensory primary sensory cortical areas for further processing (Thau et al., 2023; Torrico and Munakomi, 2023). Association prefrontal areas integrate signals from various sensory modalities to ascertain comprehensive perception driving proper responses (Romanski, 2012) that are informed by higher level cognitive analysis, formulating the potential significance of a given stimulus as juxtaposed to potential positive or adverse outcomes (Lerner et al., 2015). The limbic system structures such as the hypothalamus, amygdala and hippocampus are crucial mediators involved in stress responses by adding an emotional context to sensory experiences, as they are receiving input from sensory and cognitive pathways (Godoy et al., 2018; Herman, 2012).
The hypothalamus directs and synchronizes the neuroendocrine aspects e.g. the sympathoadrenal system and the HPA axis (Elman and Borsook, 2016; Smith and Vale, 2006). The amygdala, a key component in emotional processing, coordinates stress-related anxiety and fear (Borsook et al., 2007; Daviu et al., 2019; Elman and Borsook, 2018). Beyond the classical amygdalar circuitry (Janak and Tye, 2015), the extended amygdala e.g., the bed nucleus of the stria terminalis refines the emotional experiences (Elman and Borsook, 2018). The nucleus accumbens, a central hub in the brain's reward and reinforcement circuitry (Scofield et al., 2016), likewise substantially contributes to the affective and motivational dimension of chronic stress (Campioni et al., 2009); insula is responsible for interoception (Haruki and Ogawa, 2021), while reticular formation nuclei mediate arousal and vigilance (Arguinchona and Tadi, 2023). Simultaneously, the medial prefrontal cortex restrains stress systems in concert with the hippocampus, the brain region that is involved in memory and learning (Godoy et al., 2018; McEwen and Gianaros, 2010). Neurochemically, stress influences pivotal systems modulating mood and cognition like norepinephrine, dopamine, serotonin, glutamate and gamma-aminobutyric acid (Fuchs and Flugge, 2004; Kumar et al., 2013). Neurotransmitters’ imbalances introduced by chronic stress may become harbingers of mental disorders such as anxiety (McLaughlin and Hatzenbuehler, 2009), depression (Fuchs and Flugge, 2004) and psychosis (van Winkel et al., 2008).
4. Non-specific vs. Specific Stress Definitions
Stress definitions range from non-specific bodily responses to any type of challenge (Selye, 1950a) to a relatively specific pattern of an effector organ/cell outflow resulting from an actual-, perceived- or anticipated input discrepancy with a homeostatically-defined set point (Elman and Borsook, 2016; Goldstein, 2021). The non-specific nature of stress may be suggested by overlapping brain changes in the aforesaid corticolimbic structures (Daviu et al., 2019) that are observed in response to general categories of adverse experiences, such as emotional trauma (Elman and Borsook, 2019; Elman et al., 2018), anxiety (Chavanne and Robinson, 2021), social isolation (Lam et al., 2021; Bowirrat et al., 2023), pain (Elman et al., 2013; Elman et al., 2011) and pharmacological stimuli (Elman et al., 2012). The non-specific interpretation may be consistent with Hans Selye's stress theory, which postulates a stereotyped response to any type of challenge (Goldstein, 2021) and thus renders stress readily amenable to scientific inquiry when any laboratory-based challenge is deemed externally valid (Andrade, 2018; Selye, 1998) i.e., generalizable across various populations, stimuli, and times as well as pertinent (Holleman et al., 2020) to real-life situations (ecologic validity).
Modern scientists, however, find the non-specific perspective unnecessary reductionistic (Goldstein, 1995). The current prevailing view is that stress affects specific brain networks and systems (e.g., sensory or limbic) and other organs (e.g., heart, gut, kidneys, lungs, skin, etc.) in distinct ways, leading to a variety of stress-related conditions (Elman and Borsook, 2016). For example, mental- as opposed to physical stressors tend to activate distinct brain areas with varying magnitudes (Emmert and Herman, 1999). Furthermore, stimulation of the prelimbic cortex may play a differentiating role in the neuroendocrine responses to mental- vs. physical stressors (Jones et al., 2011). Within this theoretical framework, stress response is non-specific in terms of activating diverse responses (Selye, 1950b, 1976, 1998), whereas it is specific in the sense that a particular type of stress consistently triggers a distinct set of processes (Saab et al., 1992; Saab et al., 1993). Chronic stress can be then viewed as an intervening variable that links diverse allostatic changes with mental and physical symptoms (Goldstein, 1995; McEwen, 2007; Ulrich-Lai and Herman, 2009). Yet, the above symptoms are not solely determined by allostatic changes (McEwen, 2007) but are also influenced by various host- and environment-related factors. In other words, although stress symptoms are determined to some extent by the nature and intensity of stressors, their clinical presentation is also shaped by an individual constitution, genetic makeup, lifestyle, coping resources, state of preparedness, prior exposure to stress, and preexisting conditions (Elman and Borsook, 2019, 2022).
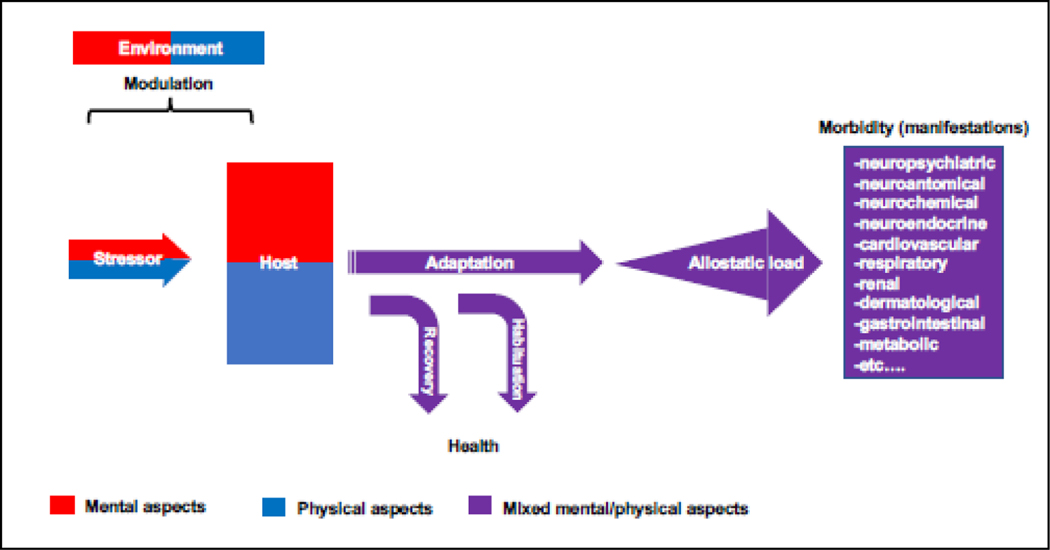
Stress thus encompasses both mental and physical components (Joseph et al., 2021) giving rise to an amalgamation of whole-organism processes that are unique for every person (Figure 2). Pain is an example of the intricate relationship between mental and physical experiences as it may originate exclusively from emotional or social sources, completely devoid of any nociceptive input (Elman and Borsook, 2018; Elman et al., 2013; Elman et al., 2011). Similarly, a slur by a co-worker (social) may elicit an emotion of rage (mental) accompanied by chest pain (physical); both are modulated by environmental factors (e.g., social support, nutrition, or toxic exposure) leading to a possible adaptation (Kalisch et al., 2017). What is more, multiple medical conditions may masquerade as a stress-related psychiatric syndromes (Welch and Carson, 2018) e.g., Cushing disease, where the overproduction of cortisol is presenting as depression and anxiety (Hinotsu et al., 2022). All other things being the same, stress may also interchange pleasant, aversive, or neutral valuation based upon the memorized or perceived contextual factors (Elman et al., 2023). Accordingly, successful coping with a stressful situation may be perceived as pleasant, rewarding, and educational echoing the commonly accepted psychological notion that active avoidance of punishment is reinforcing (Gross, 2006) and evidenced in a variety of stress-seeking behaviors such as roller coasters, automobile racing, skydiving and horror movies (Elman and Borsook, 2019). In essence, stress is a highly individualized experience that everybody understands its meaning but experiences it in a unique way. Establishment of ecological validity or even external validity is complicated because in real life it may be difficult if not impossible to specifically isolate unpredictable contributions of personal meaning from putative mental and physical components (Holleman et al., 2020).
Figure 2:
Schematic overview of the complex interplay among a stressor, host and environment. Stress is not a unitary phenomenon mediated by an isolated mechanism, (neuro)chemical or a neurotransmitter. On the contrary, this hierarchical, multidimensional entity represents integration of higher-order stressor (e.g., nature, intensity or duration), host (e.g., genes, demographics, prior exposure, personality, health) and environment (social network, nutrition, ecology) components and derived from them, lower-order mental and physical manifestations. Each of these plays a specific role within an extensive biopsychosocial system. The combination of mental and physical components gives rise to a variable mental/physical concoction of outcomes. For instance, a slur by a co-worker (mental) may elicit a sense of rage (mental) accompanied by chest pain (physical); both are modulated by social (mental) and nutritional (physical) environmental factors leading to a possible adaptation. Notwithstanding the initial adaptation, habituation or recovery, stressors by their chronic nature tend to generate a mounting allostatic load manifested in poor sleep, impaired cardiovascular function, and systemic inflammation (to name a few). Contrary to such a schematic overview, in real life, it may be difficult if not impossible to specifically account for contributions of the putative mental and physical components because a seemingly physical phenomenon e.g., pain can be solely derived from emotional or social sources in the absence of nociceptive input (Elman et al., 2013) whereas multiple medical conditions can masquerade as a psychiatric syndrome (Welch and Carson, 2018) e.g., anti-N-methyl-D-aspartate receptor encephalitis (Hinotsu et al., 2022).
To better understand stress, it may be helpful to examine distinct models based on the specific type of a stressor (e.g., sensory, cognitive, or metabolic), host (e.g., demographics, state of health and personal meaning), and environmental (e.g., support, culture, and nutrition) factors (Figure 2). Examining each model separately can provide a solid foundation for understanding its interactions with the related constituents (host and environment). A critical question, addressed here, is whether commonly used experimental stressor models e.g., public speaking, cognitive tasks, hypovolemia or glucoprivation (Elman et al., 1998; Elman and Borsook, 2019; Elman et al., 2003; Kirschbaum et al., 1993) while potentially limited by ethical considerations (non-maleficence principle) and questionable ecological and external validities, still provide valid information about the objective and subjective characteristics of stress. Despite being only one of numerous legitimate ways that "stress" can be scrutinized, these models offer several advantages. First, their validity can be confirmed through existing theoretical and empirical formulations. Second, emerging models can be compared to them as benchmarks of the validated ones (Amirkhan et al., 2015). Third, integrating selected models is likely to yield a more comprehensive methodology for conceptualizing stress phenomena (Bell et al., 2017).
5. Sifting through Stress: From Stressors to Experimental Models
A cascade of mental and physiological stress responses has been reviewed in detail elsewhere (Akil and Nestler, 2023; Forstenpointner et al., 2022), and some key elements are briefly presented here (Table 2). Stressors are stimuli or events spanning mental challenges to physical perturbations (Anisman and Merali, 1999). Stressor-evoked measurable variables are implemented in psychometrically assessed stress ratings (Frisone et al., 2021) together with indices of the sympathoadrenal system, or orchestrations of the HPA axis (Smith and Vale, 2006) allowing to systematically assess and quantify construct- and criterion-validities in the realm of stress. Test paradigms are structured and methodically designed protocols to induce and measure stress responses as exemplified by specific mental (Caruso et al., 1994; Dedovic et al., 2009) or pharmacological (Elman et al., 2012) perturbations (Brouwer and Hogervorst, 2014; Sallee et al., 2000). Such paradigms offer controlled environments, where the experimental evocation of stress reactions can be studied with precision, shedding light on the variability in validities influenced by diverse stressor stimuli. Paradigms establish sound footing for broader models (e.g., PTSD, social defeat, or the TSST) serving as scaffolding for the controlled exploration of stress (Kahn, 2002). At its core, the cornerstone of stress models’ interpretability and validity is formed by thoughtful consideration given to type of stressors and their corresponding paradigms.
Table 2.
Stressors, parameters and experimental models.
| Element | Definition | Characteristic | Purpose |
|---|---|---|---|
| Stressors (events/accidents) | Stimuli or events that trigger physiological or psychological responses. | Diverse, encompassing both mental and physical stressors e.g., academic exams, public speaking, heavy traffic or financial challenges. | Elicitation of stress responses enabling exploration of the stressor constructs |
| Evaluation parameters | Measurable variables capturing physiological or psychological changes. | Psychometrically assessed stress ratings; activation of sympathoadrenal system and the hypothalamic-pituitary-adrenal axis e.g., heart rate variability, cortisol levels, self-reported stress scores. | Systematic assessment and quantification of stress-induced reactions enabling assessment of stress models’ construct- and criterion-validities. |
| Paradigms | Structured protocols designed to systematically induce and measure stress responses. | Mental and pharmacological perturbations (e.g., Stroop, simulated job interview, yohimbine, adenosine or dobutamine). | Controlled environments for studying stress reactions experimentally; highlight variability in validity based on the nature of stressor stimuli. |
| Models | Frameworks or systems used in research to study stress under controlled conditions. | Varying validity based on the nature of stressor stimuli (e.g., PTSD, social defeat, or the TSST); shared similarities and differences in construct-, criterion-, convergent-, and discriminant-validities. | Exploration of similarities and differences in the validities of stress models; emphasis on consideration in model selection based on stressor stimuli. |
6. Integrating Validities
Stress models stretch the entire range of the experienced variable, from unconscious to an escalating degree of discomfort and suffering (Cohen, 1995). The quantitative aspect of the stress construct has been measured in numerous studies (Henn and Vollmayr, 2005; Willner, 1984) using self-ratings and more structured/comprehensive rating scales and interviews. While the validity of data obtained by such methods has been examined in student, community, and workplace populations, there remains potential for underreporting, denial, and cognitive distortions (Elman et al., 2000). Therefore, more objective assessments such as physiological responses or biomarkers criteria complement self-reported measures (Smyth et al., 1998) by quantifying the strength of correlation with self-report data (Cohen et al., 1983). This complementary approach also possesses practical importance for establishing the convergent and discriminant validity (Schuhmann et al., 2022; Westen and Rosenthal, 2003). The former validity refers to the extent to which different measures of the same construct are related (e.g., distress and anxiety); the latter validity refers to the extent to which a specific model reflects a unique construct (Table 1). By combining diverse validities, researchers and clinicians alike can gain a more comprehensive understanding of stress and its effects on mental and physical health.
7. Sympathoadrenal System and HPA Axis
Sympathoadrenal System.
A constituent of the autonomic nervous system (McCorry, 2007), the sympathoadrenal system, encompasses the sympathetic nervous system and the adrenal medulla (Cohen, 1995). The sympathoadrenal system is responsible for the instantaneous (seconds-minutes) "fight or flight"-type confrontation of a stressful situations (Godoy et al., 2018; Goldstein, 2021). Originating in the spinal cord, sympathetic nervous system innervates (via the norepinephrine neurotransmitter) various target tissues and organs to generate physiological adaptations to stress (Goldstein and Kopin, 2007). The adaptations include increased cardiac output, leading to enhanced blood flow and oxygen supply to skeletal muscles and vital organs (Joyner and Casey, 2015). Simultaneously, there is vasoconstriction to reduce blood supply to non-essential organ (Bruno et al., 2012) that can tolerate a temporary hypoperfusion without compromising vital functions or immediate survival (skin, digestive system, kidneys, reproductive system, and extremities). Also, bronchial dilation occurs to improve oxygen intake and mydriasis enhances visual acuity (Goldstein, 1995). The endocrine extension of the sympathetic nervous system, the adrenal medulla, releases mainly epinephrine (80%) and a smaller amount (20%) of norepinephrine (Carroll, 2007) into the systemic circulation. While both catecholamine hormones have similar vasoconstrictive effect, the former has broader systemic impact e.g., enhanced cardiorespiratory performance, glycogenolysis, and lipolysis (Goldstein, 1995). Negative feedback mechanisms within the sympathoadrenal system aimed at preventing overstimulation e.g., paraventricular nucleus (PVN) of hypothalamus receives feedback from the locus coeruleus to modulate the sympathetic response (Samuels and Szabadi, 2008a). Moreover, activation of presynaptic α2- adrenoceptors centrally in the hypothalamus and peripherally on sympathetic nerve terminals inhibits further release of epinephrine and norepinephrine (Bylund et al., 2008; Gothert, 1985). Remarkably, unlike certain hormones that possess inherent mechanisms to suppress their own synthesis through negative feedback loops, epinephrine, norepinephrine and catecholamines at large lack such self-regulatory capacity (Encyclopedia, 2023)
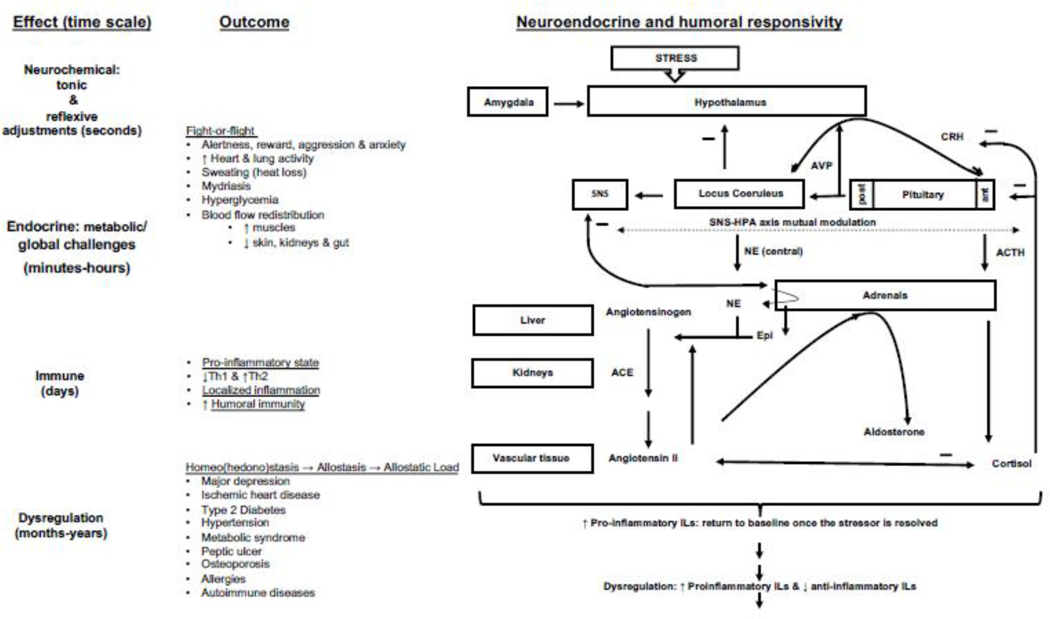
It is therefore essential to underscore the role played by complementary systems e.g., the HPA axis and other regulatory pathways, in the fine-tuning of the neurochemicals’ release and activity and maintaining their equilibrium while orchestrating the comprehensive stress response. Understanding the interactions among major stress systems helps to identify potential confounding factors that may affect the validity of experimental stress models. For example, if a stress model activates the sympathoadrenal system but not the HPA axis, it may not reliably reflect the entire range of physiological stress responses (Figure 3).
Figure 3:
Key physiological stress systems namely, the Sympathoadrenal System, HPA Axis, Renin-Angiotensin-Aldosterone System, Arginine Vasopressin and Immune System. Originating in the spinal cord, sympathetic nervous system (SNS) innervates (via the norepinephrine neurotransmitter) various target tissues and organs to generate physiological adaptations to stress (Goldstein and Kopin, 2007). The adrenal medulla, releases mainly epinephrine (80%) and a smaller amount of norepinephrine (20%) graphically displayed in the thickness of the arrow. The Sympathoadrenal System, comprising the sympathetic nervous system and adrenal medulla, initiates instantaneous "fight or flight" responses, including increased heart rate, blood flow redistribution, bronchial dilation, and heightened alertness. The HPA Axis involves helps mobilize energy reserves and suppress immune function in response to stress. These systems’ interactions range from additive to synergistic, depending on stress duration and nature. The RAAS’ hormones, aldosterone and angiotensin II regulate blood pressure and fluid balance. The Arginine Vasopressin (AVP) system plays a role in maintaining blood pressure, fluid equilibrium, and osmolarity. Both RAAS and AVP systems interact with the sympathetic and HPA systems to optimize stress responses. Stress profoundly affects immune system., immune cell activity, pro-inflammatory and anti-inflammatory cytokine release, and immune function modulation. Stress responses, while adaptive in the short term, can lead to immune dysregulation, impacting susceptibility to infections and the development of inflammatory and autoimmune conditions over time. For the clarity of presentation, the scheme was drawn out-of-scale and simplified to reduce the numbers of the displayed links and structures to those of direct relevance to the theme of this review.
Abbreviations: ACTH, adrenocorticotropic hormone; ACE, angiotensin converting enzyme; ant, anterior; CRH, corticotropin-releasing hormone; epi, epinephrine; interleukin, IL; NE, norepinephrine; post, posterior; Th1, T helper type 1; and Th2, T helper type 2
The HPA Axis is made of three components connected via reciprocal interactions namely, 1) the PVN secreting corticotropin-releasing hormone (CRH) into the hypothalamic-pituitary portal system during stress exposure; 2) the anterior lobe of the pituitary gland reacting to CRH by releasing adrenocorticotropic hormone (ACTH) into the bloodstream sensed by 3) the adrenal glands’ cortex middle fasciculata zone consequently releasing a glucocorticoid, cortisol (Chrousos, 2009; Herman et al., 2016; Smith and Vale, 2006). Cortisol facilitates stress coping by mobilizing energy reserves (via gluconeogenesis and diminished insulin’s secretion and signaling), increasing blood pressure and suppressing the immune system (Sapolsky et al., 2000; Tsigos and Chrousos, 2002). Rising plasma cortisol concentration exerts negative feedback on the hypothalamus and pituitary via the medial prefrontal cortex and hippocampus (Kinlein et al., 2015) inhibiting the release of CRH (Papadimitriou and Priftis, 2009) and ACTH (Allen and Sharma, 2023), respectively (Herman et al., 2003; Ziegler and Herman, 2002).
The integration of the sympathoadrenal system and HPA axis involves intricate feedback mechanisms synchronizing diverse brain nuclei (Forstenpointner et al., 2022; Ulrich-Lai and Herman, 2009). The amygdala and related limbic regions (Herman et al., 2016) such as prefrontal cortex and hippocampus serve as a key interface between the perceptual and cognitive appraisal of a stressor with the ensuing physiological responses (McEwen and Gianaros, 2010; Pessoa, 2010) by providing emotional (Pego et al., 2010) and contextual (Herman et al., 2016; Stanton et al., 2023) information to the PVN (Coote, 2005), which, in turn, regulates sympathetic activity and coordinates the release of HPA axis’ hormones (Ferguson et al., 2008; Herman et al., 2016; Swanson and Sawchenko, 1980). When the PVN detects a stressful or threatening stimulus, it initiates the sympathoadrenal response to stress by triggering norepinephrine release from the locus coeruleus (Berridge and Waterhouse, 2003; Valentino and Van Bockstaele, 2008) responsible for augmented body's sympathetic activity (Samuels and Szabadi, 2008b), arousal as well as attention prioritization across stressful challenges. The locus coeruleus neurons’ activity is tightly regulated by the convergence of several inputs, particularly the CRH, AVP (Berecek et al., 1984; Olpe and Steinmann, 1991), excitatory amino acids, and opioids (Berridge and Waterhouse, 2003). The interchange between the systems helps harmonize the body's overall coping means, allowing for the appropriate resources allocation and homeostasis restoration after stress adjustment (Ulrich-Lai and Herman, 2009).
The combined output of the sympathoadrenal and HPA systems culminates in a comprehensive and integrated stress response involving rapid physiological changes and sustained adjustments over time (Herman et al., 2016). Even though the specific nature of the interactions between the sympathoadrenal system and the HPA axis can vary in different stress-related circumstances (Herman and Cullinan, 1997; McEwen, 2007), cortisol modulates the release of epinephrine and norepinephrine (Wurtman, 2002) whereas catecholamines can stimulate the HPA axis (Lee et al., 2011; Lundberg, 2007) to release more cortisol (Goldstein and Kopin, 2008; Tsigos et al., 2000). Acute stress (seconds-minutes) primarily engages the sympathoadrenal system, while prolonged- (minutes-hours) or chronic stress is characterized in more complex interactions (Carrasco and Van de Kar, 2003; Godoy et al., 2018; Ulrich-Lai and Herman, 2009). By and large, three classes of interaction (co)exist between sympathoadrenal system and HPA axis, including addition, competition, and synergism (Ehrhart-Bornstein et al., 1998; Fukuhara et al., 1996).
Additive interactions involve independent but combined effects e.g., blood pressure elevation due to vasoconstriction (Goldstein, 1981) or reabsorption of sodium by the kidneys that diminishes the excretion of water (Armando et al., 2011). In a competitive interaction, epinephrine and norepinephrine suppress the activation of the HPA axis to avoid overstimulation. Synergistic interactions occur when the sympathoadrenal system and the HPA axis work together to enhance the overall stress response (Dunn and Berridge, 1990; Hirotsu et al., 2015; Ulrich-Lai and Herman, 2009). Both norepinephrine and epinephrine can amplify the activation of the HPA axis (Elman et al., 2001a; Jasper and Engeland, 1997; Ulrich-Lai et al., 2006) and increase the sensitivity of target tissues to cortisol (Herman et al., 2016; Mokuda et al., 1992; Ulrich-Lai and Engeland, 2002) whereas cortisol influences the release (Seki et al., 2018) and effects (Xiao et al., 2003) of norepinephrine and regulate the activity of enzymes involved in catecholamine metabolism (Wurtman and Axelrod, 1966). Such interaction potentiates the effects of both the sympathoadrenal system and the HPA axis, leading to an intensified physiological response to stress.
During acute stress sympathoadrenal systems’ activity may predominate against the backdrop of a relatively limited HPA axis’ activation (Rotenberg and McGrath, 2016). Conversely, over time, the HPA axis becomes prominent and the interactions between the two systems gradually shift from competitive to synergistic (de Kloet, 2003). Cortisol plays a role in both stress response modes by interacting with two types of receptors: type 1 (mineralocorticoid receptors - MR) and type 2 (glucocorticoid receptors - GR). These receptors are found in the limbic neural circuitry (Joels, 2017); Koning et al., 2019). MR regulate the baseline sensitivity or threshold of the fast stress system, thereby preventing disruption of homeostasis (Gomez-Sanchez and Gomez-Sanchez, 2014). On the other hand, GR facilitates the recovery process by dampening stress responses in stress circuits and mobilizing energy resources (de Kloet et al., 2019). Maintaining a balance between the two stress response modes is crucial for cellular homeostasis, mental performance, and overall well-being (Daskalakis et al., 2022). An imbalance, whether caused by (epi)genetic modifications or chronic stressors, can alter specific neural signaling pathways that underlie normal cognitive and emotional processes instigating allostatic load underlying medical and neuropsychiatric (co)morbidity (de Kloet, 2003; Nicolaides et al., 2000).
8. Allostatic Load
Health complications stemming from chronic stress are intricately linked to the concept of allostatic load (McEwen, 1998; McEwen and Wingfield, 2003), which emerges due to the exhaustion of homeostatic abilities to shield the body's myriad systems from cumulative wear and tear (McEwen and Gianaros, 2011). The allostatic load resulting from prolonged sympathoadrenal stimulation is marked by the disruption of negative feedback mechanisms (Goldstein, 2010) manifested in such clinical outcomes as hypertension, disrupted sleep patterns, compromised immune function, abnormalities in gastrointestinal motility, and disturbances in glucoregulatory function (Dunser and Hasibeder, 2009; McEwen, 1998; Reaven et al., 1996). That causality can operate in reverse as well (Figure 3). For instance, congestive heart failure can act as a catalyst for persistent activation of the sympathetic nervous system, adopting a misguided strategy to compensate for diminished cardiac function or to ensure adequate blood supply (Floras, 2009). This sustained release of catecholamines further erodes cardiovascular performance, that is, a detrimental feedforward interaction (Goldstein, 2021).
HPA axis dysregulation (Kinlein et al., 2015), stemming from its protracted activation, disrupts the negative feedback mechanisms governing plasma cortisol concentrations (Charney, 2004; Karatsoreos and McEwen, 2013). Diminished cortisol responses (de Kloet et al., 2006) are linked to such conditions as chronic fatigue syndrome (Powell et al., 2013) and PTSD (Kinney et al., 2023). In susceptible individuals (de Kloet et al., 2005) hypercortisolemia, conversely, heightens the risk of diabetes (Brindley, 1992), hypertension (Whitworth, 1994), osteoporosis (Czapla-Iskrzycka et al., 2022), opportunistic infections (Lionakis and Kontoyiannis, 2003), delayed wound healing (Matsuzaki and Upton, 2013), and a predisposition to autoimmune disorders (Charney, 2004) along with an array of neuropsychiatric symptomatology (Dziurkowska and Wesolowski, 2021), encompassing depression (Sahu et al., 2022; Teo et al., 2023; Varghese and Brown, 2001), anxiety (Fischer, 2021; Zorn et al., 2017), and psychosis (Elman et al., 1998; Elman et al., 2003).
9. Other Stress Systems
RAAS.
Stress systems cannot be viewed in isolation, as they are integrated within a complex network of tightly linked configurations (Figure 3), each serving a distinct purpose in the context of stress-related homeostatic and allostatic adjustments (Elman and Borsook, 2016). The RAAS system is primarily involved in the regulation of blood pressure and fluid balance (Carey, 2018; Fountain et al., 2023). Under stressful conditions, kidneys release the renin enzyme (Persson, 2003), which cleaves angiotensinogen in the liver to produce angiotensin I (Benigni et al., 2010). Angiotensin I, converted to a potent vasoconstrictor, angiotensin II by the angiotensin-converting enzyme (ACE) in the blood vessel tissue (Timmermans et al., 1993), acts on the exterior layer of the adrenal cortex, the zona glomerulosa, to stimulate the release of aldosterone, which promotes the reabsorption of sodium and water in the kidneys to increase blood volume and pressure (Fountain et al., 2023). Reciprocal interactions among the sympathoadrenal system, HPA axis, and the RAAS add further optimization to coordination of stress coping mechanisms (Atlas et al., 1981; Gideon et al., 2020; Murck et al., 2012). Norepinephrine, epinephrine as well as the HPA axis’ components, ACTH (Ganong, 1981; Hu et al., 1992; Kopp and DiBona, 1986) and cortisol (Skov et al., 2014) can enhance the RAAS with consequently elevated levels of angiotensin I and angiotensin II. In a feedforward loop, angiotensin II stimulates the sympathoadrenal system (King et al., 2013) and the release of CRH (Ganong and Murakami, 1987). Thus, the sympathoadrenal system, HPA axis and RAAS work in concert to mobilize energy reserves, increase cardiovascular function, enhance alertness; all support the body's ability to cope with immediate challenges (Figure 3). In contrast, protracted activation of these systems during chronic stress results in systematic disintegration contributing to long-term allostatic effects on blood pressure, fluid balance, cardiovascular and emotional health (Herman et al., 2016; Murck et al., 2003).
The AVP system likewise controls blood pressure, fluid equilibrium and osmolarity via osmotic and non-osmotic (373467) mechanisms e.g., hypovolemia, emotional distress or hypoxia (Cuzzo et al., 2023; Elman et al., 2003; Soto-Rivera, 2017). Osmoreceptors in the hypothalamus detect rises in solutes’ concentration and trigger AVP release from the posterior pituitary gland (Cuzzo et al., 2023); this process is enhanced by catecholamines, cortisol and angiotensin II (Leibowitz et al., 1990). AVP causes water reabsorption in the kidneys, leading to concentrated urine and water retention; AVP also augments CRH release and activity (McCann et al., 2000; Stojiljkovic et al., 2022). In this intricate arrangement, the vasoconstrictive attributes of catecholamines, cortisol and angiotensin II (Kanaide et al., 2003; Riad et al., 2002; Ullian, 1999), work in harmony to complement AVP's actions in not only constricting blood vessels, but also enhancing water retention (Cuzzo et al., 2023) thus contributing to the maintenance of blood volume and pressure during instances of heightened sympathetic activity while adjusting to changing physiological demands (Figure 3). In certain pathological conditions, such as heart failure, kidney dysfunction, or chronic hypertension, the AVP system and the RAAS may become disintegrated reflected in excessive water retention, electrolyte imbalances, and increased blood pressure (Ames et al., 2019; Ma et al., 2022; Ma et al., 2010).
Oxytocin is commonly recognized as a stress-relieving neuropeptide (Takayanagi and Onaka, 2021) via central and peripheral processes (Etgen, 1995; Ho and Blevins, 2013; Reichel, 2021) with extensive ramifications for social affiliations (Baribeau and Anagnostou, 2015; Insel, 2003), reproduction (Ivell et al., 1997; Lippert et al., 2003), anti-inflammation (Friuli et al., 2021; Yuan et al., 2016), analgesia (Nishimura et al., 2022), antioxidation (Alanazi et al., 2020; Buemann et al., 2021), metabolism (Iovino et al., 2023), cognition (Erdozain and Penagarikano, 2019), mental health (Cochran et al., 2013) and overall wellbeing (Carter et al., 2020). It is structurally alike AVP (Baribeau and Anagnostou, 2015) and both hormones are released together from the posterior pituitary (Christ-Crain and Ball, 2000). While AVP and oxytocin exert a degree of functional antagonism regarding the HPA axis (Neumann et al., 2000) and sympathoadrenal activity (Grewen et al., 2005; Uvnas Moberg et al., 2019) moderated by sex (Carter and Perkeybile, 2018; Jirikowski et al., 2018; Love, 2018), action sites (Neumann, 2002), plasma concentrations (Song and Albers, 2018) or contexts (Carter et al., 2020) they can also act as agonists at each other’s receptors yielding potentially additive or synergistic effects (Song and Albers, 2018).
Immune System.
In addition to their pivotal roles in adaption to stress and in the maintenance of blood pressure and fluid balance, neuroendocrine systems also profoundly affect immune function (Chrousos and Gold, 1992; Sekaninova et al., 2020; Tsigos et al., 2000). Acute stress favors humoral immunity by down-regulation of T helper 1 cells and up up-regulation of T helper 2 cells-mediated cytokine activity (Assaf et al., 2017; Rajaei, 2022). The neuroendocrine-immune crosstalk (Tian et al., 2012) is mediated by interleukin type cytokine proteins (Justiz Vaillant and Qurie, 2023; Liu et al., 2021) that are secreted from macrophages, T cells, T regulatory cells, B cells, dendritic cells, as well as non-immune endothelial cells and fibroblasts (Zhang and An, 2007) and can have both pro- and anti-inflammatory properties (Godinho-Silva et al., 2019; Tian et al., 2012). Stress-induced activation of the sympathoadrenal system predominately (Koldzic-Zivanovic et al., 2006) amplifies proinflammatory pathways engaging interleukin (IL) 6 and 8 (Goebel et al., 2000; Steensberg et al., 2001; van der Poll and Lowry, 1997b). Such responses may be adaptive from a phylogenetic standpoint as they are coping with potential injuries or infections during a stressful event. Yet there are also balancing effects including suppression of the tumor necrosis factor-alpha (TNF-α) and IL-1 beta (IL-1β) proinflammatory- (Van der Poll and Lowry, 1997a) and enhancement of anti-inflammatory IL-10 (van der Poll et al., 1996a; Van der Poll and Lowry, 1997a) signals by epinephrine. Proinflammatory ILs released during immune responses stimulate hypothalamus to increase AVP and the HPA axis’ activity (Bethin et al., 2000; Chikanza et al., 2000), leading to cortisol surge that dampens proinflammatory cytokines like IL-6, TNF-α and IL-1β (Dong et al., 2018).
Furthermore, proinflammatory ILs such as IL-6, TNF-α, IL-1β and IL-1 stimulate the RAAS system (Bataillard et al., 1992), leading to increased angiotensin II production (Andreis et al., 1992; Flesch et al., 2003; Gurantz et al., 1999; Senchenkova et al., 2019). Angiotensin II, in turn, contributes to the release of additional proinflammatory ILs (OĽeary et al., 2016; Pacurari et al., 2014), promoting inflammation and immune activation, which is conspicuous in conditions like hypertension and ischemic heart disease (Amin et al., 2020; Satou et al., 2018). IL-producing immune cells express AVP receptors (Baker et al., 2003) that might decrease proinflammatory cytokines (Russell et al., 2013) e.g., IL-6, TNF-α and IL-1β (Boyd et al., 2008; Park, 2015; Zhao and Brinton, 2004) and affect the migration of immune cells to specific sites in the body (Wiedermann et al., 2018).
Ultimately, the types of ILs released, and the type of immune cells involved, and their function determine the nature of the response, whether acute enhancement, diminution, or dysregulation, depending on the balance of the intricate interactions (Justiz Vaillant and Qurie, 2023). All in all, acute neuroendocrine effects on the immune response involve mobilizing immune system to fight infections, and aid tissue repair whereas prolonged stress may dysregulate immune function including an overproduction of proinflammatory ILs and an underproduction of anti-inflammatory ILs (Figure 3) manifested in susceptibility to infections, and the development of inflammatory and autoimmune conditions (Dhabhar, 2009; Segerstrom and Miller, 2004).
10. Construct and Criterion Validity
Construct validity: Validated psycho-diagnostic and psychometric tools.
Due to their standardized, objective, and comprehensive measurements of psychological stress (Blevins et al., 2015; Weathers et al., 2018) many psycho-diagnostic/metric tools (Osorio et al., 2019; Shankman et al., 2018) offer suitable means for evaluating the construct validity of stress models (Crosswell and Lockwood, 2020). Among others, tools like the Structured Clinical Interview for DSM-5 (SCID-5) Disorders (Shabani et al., 2021), Clinician-Administered PTSD Scale (CAPS) for DSM-5 (Back et al., 2022; Elman and Borsook, 2022), PTSD Checklist (PCL) for DSM-5 (Kramer et al., 2023) and even self-ratings (Masood et al., 2012; Thornorarinsdottir et al., 2019) have undergone rigorous testing to enable the inquiry into emotional, cognitive, and behavioral dimensions of stress providing a nuanced understanding of their construct validity (Aguilo Mir et al., 2021; Amirkhan, 2018). The yielded quantitative data allows statistical testing to determine the association between stress models and psychological constructs and to compare stress outcomes with available norms or benchmarks for the specific psychological constructs being assessed (Elman et al., 2009; Elman et al., 2018). Standardization of the assessments facilitates their meaningful comparisons and conclusions as they are applied consistently across different individuals and settings. Psycho- diagnostic/metric tools likewise provide objective measurements of psychological constructs related to stress, such as anxiety and depression (Elman et al., 2010; Green et al., 2017). Importantly, many psychodiagnostic tools have clinical relevance and are used in diagnosing and treating stress-related mental health conditions (Weathers et al., 2018). By evaluating the construct validity of stress models with tools commonly employed in clinical practice, researchers can connect theoretical stress concepts with real-world applications rendering these tools a crucial element in both clinical settings and research environments.
Criterion validity: The sympathoadrenal system and HPA axis.
Criterion validity refers to the extent to which a model is capable of accurately and consistently predicting or correlating with a particular criterion or outcome of interest (Piedmont, 2014). As alluded above, the suitability of the sympathoadrenal system and HPA axis activations as criteria, albeit not the exclusive ones (Levy et al., 2021), for assessing the validity of stress models stems from their central role in the physiological and psychological response to acute and chronic stress (Kvetnansky et al., 1995; Smith and Vale, 2006). There are many additional reasons as to why the sympathoadrenal system and HPA axis are regarded as key criterion validity characteristics for stress models.
These systems are widely recognized in the scientific and clinical communities as biomarkers of stress (Piazza et al., 2010). In terms of practicality, catecholamines and cortisol are readily quantifiable in blood, saliva, or urine (Beerda et al., 1996; El-Farhan et al., 2017). In the realm of physiology, sympathoadrenal system mediates immediate, ‘fight-or-flight’ responses (Carter and Goldstein, 2015) while the HPA axis regulates the sustained response to stress (Herman et al., 2016). Stress models that incorporate both systems can account for the temporal dynamics of the stress response, making them more comprehensive and physiologically relevant. On the clinical level, the sympathoadrenal system and HPA axis are key neuroendocrine pathways determining stress resilience or vulnerability (Charney, 2004). Valid stress models could be instrumental for stress vulnerability screening with primary and secondary prevention implications (Elman and Borsook, 2019). From pathophysiological perspective, since the sympathoadrenal system and HPA axis are associated with a range of stress-related psychiatric and medical disorders (Bjorntorp et al., 2000; Maes et al., 1991), valid stress models may capture specific dysregulations to provide mechanistic and therapeutic insights. Notwithstanding the sympathoadrenal system and HPA axis frequent use as primary criteria for stress model validation, a comprehensive approach to stress modeling ought to consider multiple criteria to ensure understanding of the complex biopsychosocial dimensions of stress. Thus, the choice of criterion validity characteristics needs to align with the aims of research and the aspects of stress being investigated.
Due to the complexity of the stress mechanisms and the interactions among its various facets, it would be possible to generate countless volumes of scientific and clinical work depicting stress-related biopsychosocial effects and alterations. Similarly, when considering the organ systems affected by stress in comparison to non-stressed subjects, the list seems exhaustive, encompassing every part of the body from head to toe. For the present discussion, we address frequently employed diverse and representative stress models exemplifying their commonalities, disparities, and diverse validities namely, the PTSD, social defeat, TSST, MAST, Stroop test, infection, hypovolemia, glucoprivation, heat, sleep deprivation, pain, adenosine, dobutamine and yohimbine (Table 3).
Table 3:
Abridgment of representative stress models
| Origin | Duration | Cause | Validity | Comments | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Acute | Chronic | Natural | Man-made | Construct (measure) | Convergent | Discriminant | Criterion | ||||
| HPA axis | Sympat hoadrenal | ||||||||||
| Mental | — | PTSD (e.g., torture motor vehicle accident) | X | X | Reliving traumatic event(s) measured via Clinician-Administered PTSD Scale, the PTSD Checklist (Mueser et al., 2001), Civilian (Green et al., 2017) and Combat (Beresford et al., 2021) Mississippi Scale etc... | Horror, anger guilt, or shame; diminished value of natural reinforcers and unhappiness (American Psychiatric, 2022) | Pathophysiological model of chronic stress (Elman and Borsook, 2019) | ↓ (Fischer et al., 2021; Gola et al., 2012) | ↑ (Fu, 2022; Pervanidou et al. 2007), but see (Videlock et al., 2008); sympat hetic hyperarousal is the key feature (American Psychiatric, 2022) | Laboratory animals’ models may not properly capture the complexity of the syndrome (Videlock et al., 2008) | |
| — | Social defeat | — | X | The Social Defeat Scale, (Gilbert and Allan, 1998) | Bullying, subordin ation (Bjorkqvist, 2001), burnout, work-related stress (van der Molen et al., 2020), aggression and humiliation (Bjorkqvist, 2001; Rohde, 2001); debating with a member of the opposite gender (Bjorkqvist, 2001; Rejeski et al., 1989)“ residentintruder“ procedure in animals (Koolhaas et al., 2013) | Neuropsyc hiatric disorders e.g., major depression, generalized anxiety disorder and schizophre nia (Bjorkqvist, 2001; Laviola et al., 2004; Rohde, 2001) | ↑ (Cannizzaro et al., 2020; Iob et al., 2021; Laceulle et al., 2017) | ↑ (Ghaddar et al., 2014; Nakatake et al., 2020; Rajalingam et al., 2021) | More studies on concordance between human and animal research are needed (Bjorkqvist, 2001) as human studies are usually retrospective or cross-sectional | ||
| Trier social stress test | — | — | X | Self-reported acute stress (Hellham mer and Schubert, 2012; Mohiyeddini et al., 2013) | interview preparation, public speech and mental arithmetic task (Frisch et al., 2015); ↑ anxiety and insecurity (Hellhammer and Schubert, 2012) | Robust and reliable neurobiological effects notwithstanding the psychosocial nature of the task (Allen et al., 2017) | ↑ (Gabrys et al., 2019; Hellhammer and Schubert, 2012) | ↑ (Bremner et al., 1996) | Ecological validity is good for Western cultures, external validity for non-Western remains to be examined | ||
| Maastricht acute stress test | — | — | X | Self-reported acute stress (Smeets et al., 2012; van Ruitenbeek et al., 2021); the State-Trait Anxiety Inventory (Shilton et al., 2017) | Elements of the Trier Social Stress Test, the Cold Pressor Test, stress of social evaluation (Smeets et al., 2012) | Combination of the Trier social stress test and the Cold Pressor Test is unique | ↑ (Bali and Jaggi, 2015) | ↑ (Bali and Jaggi, 2015; van Ruitenbeek et al., 2021) | Limited ecological validity of the Cold Pressor Test | ||
| Stroop test | — | — | X | Self-reported acute stress (Renaud and Blondin, 1997) | Self-reported anxiety (Tulen et al., 1989); working memory (Tulen et al., 1989); information processing e.g., attention (Parris et al., 2022), speed (Perianez et al., 2021) and parallel disturbance (Herd et al., 2006) | Consistent engagement of the anterior cingulate- and dorsolateral prefrontal cortices (Milham et al., 2003) | ↑ (Compton et al., 2013; Young Kuchenbecker et al., 2021) | ↑ (Huang et al., 2021; Renaud and Blondin, 1997; Vazan et al., 2017) | Multiple trials are required in order to elicit stress responses (Renaud and Blondin, 1997) | ||
| Physical | Infection | X | X | X | “Post-COVID Stress Disorder” ≅ PTSD (Tucker, 2021); Holeboard open field apparatus for anxiety-like behavior (Lyte et al., 2006) | Generalizable across various types of pathogens (Gareau et al., 2011) | Memory dysfunction (Gareau et al., 2011) | ↑ (Dunn, 1993a, b) | ↑ (Dunn, 1993a, b; Dunn et al., 1989) | Paucity of human studies; the construct validity of the COVID-induced stress is questiona ble given potential environmental impacts | |
| Hypovolemia (e.g., dehydration or blood loss) | — | X | X | Anxiety (self-reported or observed) ((Medline Plus, 2021) | Agitation, confusion and fatigue (Medline Plus, 2021) | Initially specific stress response (activation of the sympathon eural system), which becomes generalized following deterioratio n (Goldstein, 1995) | ↑ (Gann, 1979; Lilly et al., 1983) | ↑ (Bond and Johnson, 1985) | Poor construct validity | ||
| Glucoprivation (hypoglycemia) | — | — | X | Self-reported distress (Breier, 1989; Elman et al., 2004) | Whole body level hormonal response (Kerr et al., 1989; Schwartz et al., 1987) | Hunger, counterreg ulatory hormones (Breier, 1989; Muneer, 2021) | ↑ (Breier, 1989; Elman et al., 1998) | ↑ (Breier, 1989; Elman et al., 2004) | Acccomp anied by hunger and tiredness | ||
| Heat | X | X | X | Subjective heat stress question naire (Beckmann, 2021 #738; 26411664}. Objective quantification of the environmental stress-, personal stress- and heat strain indices factoring in ambient and core temperature, relative humidity, solar radiation and heart rate (Garzon-Villalba et al., 2017; Ramphal-Naley, 2012). | Heat-induced pain (Elman et al., 2018) | Heat exhaustion (Gauer and Meyers, 2019), hyperventilation (Tsuji et al., 2016), syncope, edema, rash (Howe and Boden, 2007), (Wilson et al., 2014) and stroke (Morris and Patel, 2023) | ↑ (Brenner et al., 1998; Follenius et al., 1982; Wang et al., 2015) | ↑ (Boonruksa et al., 2020; Cheshire, 2016; Cramer and Jay, 2016) | Good ecologic validity as heat is part of everyday life (Kunz-Plapp, 2018) external validity is questionable given cross-cultural temperature preference variability (Havenith, 2020) | ||
| Sleep deprivation | X | X | X | Various stress questionnaires (Gardani et al., 2022) e.g., a global measure of perceived stress (Cohen et al., 1983); Depressi on Anxiety Stress Subscale (Lovibond and Lovibond, 1995) and Undergra duate Stress Question naire (Schlarb et al., 2017), the stress component of the Patient Health Questionnaire (Schlarb et al., 2017) | Hypertension, glucoregulatory abnormalities, cardiovascular events, depression and anxiety (Hanson and Huecker, 2023) | Diminished alertness, excessive sleepiness, accidents (Colten et al., 2006) | ↑ (Nollet et al., 2020; Tsai et al., 1989) | Inconsistent findings; both ↑ (Lusardi et al., 1999; Tochikubo et al., 1996) and no change reported (Kato et al., 2000) | Good ecologic and external validity in the face of questionable criterion validity | ||
| Pain | ≅ PTSD due to persistent relieving of stress (26748087) | X | X | Various stress questionnaires e.g., Cohen’s Perceived Stress Scale (Vinstrup et al., 2020) | Neurobiological and clinical overlap with emotional pain e.g., depression, anxiety and PTSD (Borsook et al., 2016; Elman et al., 2013; Elman et al., 2011) | The involvement of the neural sensory apparatus (Elman and Borsook, 2016); pain-induced pleasure or algophilia (Elman and Borsook, 2016) | Initially analgesic (Hannibal and Bishop, 2014); chronically enhances pain (Griep et al., 1998; Tennant and Hermann, 2002) | Initially analgesic; later evolves to enhance pain (Taylor and Westlund, 2017; Tsigos et al., 1993) | Limited criterion validity; limited external validity e.g., cross cultural variability in perceived childbirth pain; offset of pain from daggers and hooks deeply penetrating the body from being a participant in a sacred ceremony (Elman and Borsook, 2016) | ||
| Pharmacological agents | Adenosine | — | — | X | Indirect evidence cardiac effects are similar to those of mental stress (Gottlieb et al., 2014) | — | An alternative for cardiac stress test (O’Keefe et al., 1992); causes transient AV block; reduces blood flow to ischemic areas i.e., coronary steal (Aetesam-Ur-Rahman et al., 2021) in the face of increased cardiac perfusion (Yimcharoen et al., 2020) | ↓ (Chamey et al., 1985) | ↓ (Richardt et al., 1989) | Poor validity as a stress model | |
| Dobutamine | — | — | X | Has not been methodically assessed for self-reported physical and mental stress (Wagner et al., 1996) | — | Utility for treatment of heart failure (Pickworth, 1992); may be used as cardiac stress test (Elhendy et al., 2002) | ↑ (Taylor, 1998) | ↓ (Velez-Roa et al., 2003) | Question able validity as a stress model | ||
| Yohimbine | — | — | X | Self-reported stress (Elman et al., 2012) and psychom etric ratings (Umhau et al., 2011) | Anxiety (Tam et al., 2001; Vasa et al., 2009), fear (Kausche et al., 2021) and pain (Ji et al., 2022) | FDA approved for the treatment of the Erectile Dysfunction (Tam et al., 2001) | ↑ (Fricke et al., 2023; Grunhaus et al., 1989; Price et al., 1986) | ↑ (Biaggioni et al., 1994; Petrie et al., 2000; Swann et al., 2013; Tanaka et al., 2000) | Requires safety monitoring for potential hypertension, tachycardia, tachypnea, arrhythmia, bronchos pasm and nausea (Landis and Shore, 1989; Lefton, 2023; Linden et al., 1985; WebMD, 2023) | ||
11. Evaluating the Validity of Stress Models
As displayed in Table 3, stress models can be classified into categories (MedlinePlus, 2021; SAMHSA, 2023) baased on the disposition (mental vs. physical), duration (acute vs. chronic), and causes (natural vs. man-made). Mental stress models target cognitive and emotional faculties (Elman et al., 2018) via tasks eliciting potential psychological discomfort (e.g., public speaking, cognitive challenge, or emotionally upsetting images or sounds). Physical stress models likewise encompass potentially uncomfortable exposures primarily in the somatic domain e.g., hot or cold temperatures (Elman et al., 2018), glucoprivation, hypovolemia (Elman et al., 2012; Elman and Breier, 1997; Elman et al., 2004; Triedman et al., 1993) and other types of homeostatic perturbations. Another critical dimension of stress modeling relates to the duration of the stress exposure. Acute stress models replicate brief, intense stressors, akin to sudden life-threatening situations vs. chronic stress models mimicking the protracted, enduring stressors experienced over an extended period (Elman and Borsook, 2019; Elman et al., 2013; Elman et al., 2009) and resembling the stressors encountered in prolonged adversity or chronic illness (Borsook et al., 2016).
Natural stress models pertain to stressors that occur spontaneously in the environment, such as natural disasters, climate-related stressors, or biological strains whereas man-made stress models involve stressors like social conflicts, job-related stress, or exposure to pharmacological stress-inducing agents (Elman et al., 2012). Man-made stress is obviously more predictable and controllable while natural disasters may arise spontaneously from the environment and can be challenging to replicate in a laboratory setting. The intentional creation of stressors in man-made models can lead to intense and potentially harmful emotional impact, as some researchers tend to push boundaries to study extreme reactions (Bocchiaro and Zimbardo, 2017). Such deliberate infliction of stress raises poignant ethical questions about the potential harm to participants and the boundaries of research practices (Zimbardo, 1973). In contrast, natural stressors can be highly unpredictable and uncontrollable (Anisman and Merali, 1999), making them difficult to study in controlled research settings. This unpredictability can likewise lead to heightened anxiety and distress (Bromet, 2018).
11.1. Clinical Conditions
PTSD.
Due to its essential diagnostic criteria, i.e., experience of an extreme traumatic stressor and persistent reliving of the traumatic event for months or years, PTSD may serve as a naturalistic model of chronic stress (Elman et al., 2005; Elman et al., 2011; Hopper et al., 2008). Here, the use of the term "model" was extended to encompass the reverse translational strategy (Venniro et al., 2020) applying clinical findings to preclinical or basic research settings (Dudek et al., 2021). As a corollary, the PTSD reverse translational model helps to bridge the gap between PTSD clinical observations and experimental studies by integrating clinical findings into experimental designs (Sial et al., 2016) to facilitate a more comprehensive and multidisciplinary approach to studying the impact of environmental factors, neurobiological mechanisms of chronic stress and related disorders, and therapeutic approaches (DePierro et al., 2019).
Construct validity of the PTSD model is defined by specific diagnostic criteria (e.g., re-experiencing, avoidance, negative alterations in cognition and mood, and heightened arousal) providing a clear and reliable diagnostic framework (American Psychiatric, 2022) for a SCID-5- or CAPS-driven structured interview. The latter is the gold standard for determining PTSD’s construct validity including psychometric data on the severity ratings of each diagnostic criterion (Elman et al., 2009). Being based on self-reports, the PCL gleans information on subjective aspects of the PTSD construct. The Mississippi Scale’s (Green et al., 2017) value in the differentiation and specification of the PTSD construct’s validity as a chronic stress model lies in its ability to tailor the assessment to the unique stressors faced in combat vs. civilian traumatic events.
PTSD is associated with significant physiological changes that support its criterion validity as a chronic stress model including the sympathoadrenal (Pan et al., 2018), HPA axis (Schindler-Gmelch et al., 2023) RAAS (Khoury et al., 2012; Seligowski et al., 2021), AVP (Difede et al., 2023; Lago et al., 2021) and immune dysregulation (Katrinli et al., 2022). Yet, the data in this regard is not entirely consistent (Fu, 2022; Pervanidou et al., 2007; Videlock et al., 2008) and even a diminution of the HPA axis’ activity being frequently reported (Fischer et al., 2021; Gola et al., 2012; Szeszko et al., 2018). PTSD’s convergent validity is supported by common co-occurrence with other stress-related conditions, such as major depression, anxiety disorders (Flory and Yehuda, 2015; Grinage, 2003), anhedonia, and affective flattening (Blum et al., 2022; Elman and Borsook, 2019). These comorbidities point to the wide-ranging impact of chronic stress on mental health. On the other hand, the unique aspects of PTSD as a pathophysiological model of chronic stress include the specific etiology (traumatic exposure), triggers and some symptoms profile (e.g., flashbacks and distress from trauma cues exposure). In short, by showcasing the interrelation of mental and physiological processes in the context of chronic stress, PTSD possesses strong construct-, convergent-, and discriminant-validities as a chronic stress The criterion validity is somewhat weaker given the inconstancies of the neuroendocrine data.
Social Defeat.
Social defeat entails prolonged exposure to social adversity such as subordination or humiliation (Bjorkqvist, 2001). As a model of chronic stress, it has been rather extensively investigated in both humans (Bjorkqvist, 2001) and laboratory animals (Toyoda, 2017). The Social Defeat Scale is a standardized psychometric tool assessing the construct of social defeat including items related to helplessness, hopelessness, demoralization, and humiliation (Gilbert and Allan, 1998; Lincoln, 2022). Chronic social defeat is associated with enhanced sympathoadrenal (Ghaddar et al., 2014; Nakatake et al., 2020; Rajalingam et al., 2021) and HPA axis’ (Cannizzaro et al., 2020; Iob et al., 2021; Laceulle et al., 2017; Marini et al., 2006; Niraula et al., 2018) activity as well as enhanced RAAS’ (Berton et al., 1999; Brouillard et al., 2019)-, AVP (Litvin et al., 2011; Wotjak et al., 1996) and immune system’s (Ishikawa et al., 2021) responses demonstrating criterion validity. As is the case for other stressors, neuroendocrine alterations initially contribute to adaptive increases in blood pressure and water retention. However, continuous state of defeat leads to various ailments, particularly cardiovascular problems as well as inflammatory states and altered immune cell distribution (Samuels et al., 2023).
Chronic social defeat is often linked to related constructs such as bullying (Bjorkqvist, 2001), burnout, work-related stress (van der Molen et al., 2020), aggression, humiliation (Bjorkqvist, 2001; Rohde, 2001), discrimination (Lincoln, 2022) and specific social situations like debating with a member of the opposite gender (Bjorkqvist, 2001; Rejeski et al., 1989). The presence of associations between social defeat and these related constructs supports convergent validity capturing essential aspects of chronic social stress. Animal models, such as the "resident-intruder" procedure (Koolhaas et al., 2013), are used to model social defeat situations in laboratory settings through aggressive encounters (Munshi et al., 2022). Parallel findings in human and animal studies enhance the convergent validity (Morais-Silva et al., 2019). While major depression, generalized anxiety disorder, and schizophrenia (Bjorkqvist, 2001; Laviola et al., 2004; Rohde, 2001; Selten et al., 2013) clinical presentation establishes that they may share some symptoms with social defeat in terms of hopelessness, social withdrawal and emotional numbing, their distinct diagnostic criteria and symptom profiles position social defeat as a distinctive model of chronic social stress pointing to the discriminant validity.
11.2. Laboratory-Based Stress Tests
The TSST.
While challenges introduced by laboratory-based procedures (e.g., TSST) may lack ecological validity in representing daily life or real-world events (Allen et al., 2017), they hold mechanistic significance as their mental and physiological effects are like those produced by authentic stressors (Bamert and Inauen, 2022). The TSST is a popular laboratory-based procedure for induction of acute psychological stress through two structured challenges viz., job interview and math exercise (Frisch et al., 2015). The former component may be extended to other type of challenges within the framework of the Trier Mental Challenge Test e.g., time pressure or loud (75 dB) noise (Kirschbaum, 1991) adapted for use during positron emission tomography or functional magnetic resonance imaging as a part of the Montreal Imaging Stress Task (Dedovic et al., 2005).
After a short (minutes) preparation subjects participate in a simulated job interview striving to appear qualified applicants to an unresponsive panel of “experts.” An unexpected cognitive challenge in the form of math questions is added to the social evaluation and it enhances the overall stress experience (Allen et al., 2017; Bauerly et al., 2019). Individuals subjected to the TSST consistently report increased levels of stress during the task (Hellhammer and Schubert, 2012; Mohiyeddini et al., 2013) i.e., construct validity. Moreover, the TSST reliably triggers physiological responses including the heightened activity of the sympathoadrenal system (Bremner et al., 1996), HPA axis (Gabrys et al., 2019; Hellhammer and Schubert, 2012), RAAS (Gideon et al., 2020; Gunes Ozunal, 2021), AVP (Spanakis et al., 2016; Tabak et al., 2022) and immune (Allen et al., 2014) systems, supporting the criterion validity of neuroendocrine and immune changes characteristic of acute stress. The TSST’s convergent validity is evident in the consistent physiological and psychological responses across the interview preparation, public speaking, and mental arithmetic components (Frisch et al., 2015) in conjunction with the concurrent anxiety and insecurity (Hellhammer and Schubert, 2012). Despite the psychosocial nature of the TSST, its effects extend beyond the psychological realm to robust and reliable neurobiological changes e.g., growth hormone and prolactin (Kirschbaum et al., 1993) showing discriminant validity as the TSST captures unique aspects of acute stress beyond psychological dimensions (Allen et al., 2017).
The TSST faces certain limitations. Extraneous factors, such as the gender composition of the panel evaluating participants and nonverbal cues seem to influence test results, potentially compromising its ecological validity (Allen et al., 2014; Allen et al., 2017; Narvaez Linares et al., 2020). Moreover, the TSST's design combines different types of stressors, making it challenging to isolate which specific aspect of the test drives the physiological response (Allen et al., 2017). Furthermore, the test does not appear to trigger distinct physiological stress responses based on individuals' personality factors (Gaab et al., 2005). Even so, the TSST remains a valuable tool for studying the cumulative effects of acute psychological stressors in a controlled laboratory setting.
The MAST is an experimental acute stress model involving submergence of a hand in ice water for varying controlled durations (not exceeding 90 seconds, in total over ten minutes). Between submergence trials, subjects perform math tasks with negative feedback from an observer in case of a mistake (Shilton et al., 2017; Smeets et al., 2012). The MAST protocol consistently demonstrates construct validity via self-reports of acute stress (Smeets et al., 2012; van Ruitenbeek et al., 2021) and the State-Trait Anxiety Inventory (STAI) measuring psychological stress response (Elman et al., 2010; Karlsgodt et al., 2003) associated with the MAST (Shilton et al., 2017). The MAST effectively triggers activation of the sympathoadrenal system and the HPA axis evident in significant increases in blood pressure, alpha-amylase (Takai et al., 2004) and cortisol levels (Le et al., 2021; Shilton et al., 2017; Smeets et al., 2012) that is to say, criterion validity. The convergent validity of MAST is supported by the common components with other established stress models, such as the TSST and the Cold Pressor Test (Smeets et al., 2012) as well as the exposure to a challenging task in the presence of an observer (Quaedflieg et al., 2013). Yet again, the combination of both components to segregate cognitive and physiological aspects is unique; hence is the discriminant validity.
The Stroop Test.
Because people are accustomed to reading words rather than identifying the colors of the ink they are written in (Prevor and Diamond, 2005), the Stroop test creates cognitive conflict with consequent mental stress by presenting color names written in incongruent ink colors (Jensen and Rohwer, 1966). This stress construct is elicited by psychometric scales as subjects struggle to complete the task (Renaud and Blondin, 1997; Tulen et al., 1989). During the test, subjects consistently increase sympathoadrenal activity evident in the heart rate, heart rate variability and skin conductance findings (Huang et al., 2021; Renaud and Blondin, 1997; Vazan et al., 2017) together with activation of the HPA axis (Compton et al., 2013; Young Kuchenbecker et al., 2021), which is the evidence of criterion validity.
The Stroop test also demonstrates convergent validity by affecting various psychological processes including anxiety, working memory, complex information and attention and speed of processing (Herd et al., 2006; Parris et al., 2022; Perianez et al., 2021; Tulen et al., 1989). Engagement of the anterior cingulate- and dorsolateral prefrontal cortices by Stroop is a consistent finding on neuroimaging studies (Milham et al., 2003; Spaniol et al., 2009). While these areas are associated with cognitive control and conflict resolution, their consistent activation in Stroop performance distinguishes it from other stress models that may involve different neural pathways or regions (discriminant validity).
11.3. Biological Stressors
Infection.
Stress can both predispose and be caused by infections (Cohen et al., 1993; Cohen and Williamson, 1991; Liu et al., 2017). Above and beyond the direct causative effects, pandemics stressfully affect entire nations due to economic turmoil, social chaos, and isolation (Maragos et al., 1998) to the extent that a variant of PTSD may ensue viz., post-pandemic stress disorder (Laskawiec et al., 2022). The construct validity of the infection model may be derived from its propensity to induce stress responses in individuals through exposure to infectious agents as measured with conventional psychometric tools e.g., the Perceived Stress Scale (Danilescu et al., 2022) or anxiety-like behavior in animal models (Lyte et al., 2006). The criterion validity is supported by the activation of the sympathoadrenal system, HPA axis (Dunn, 1993b; Dunn et al., 1989) and AVP (Jochberger et al., 2009) as well as RAAS’ dysregulation (Li et al., 2003; Vitiello and Ferrara, 2020). Convergent validity is established by the model's generalizability across various types of pathogens (Gareau et al., 2011) and its association with negative affective states e.g., depression and anxiety (Adams et al., 2008) and the ability to impair memory leading to dementia (Janbek et al., 2023). The infection model demonstrates discriminant validity by engaging specific cognitive processes and sensory experiences related to the type of infection aside from immunological mechanisms. For example, individuals with pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections may experience obsessions and compulsions, which is a distinct symptom associated with the respective bacteria (Swedo et al., 1998).
Also, a lipopolysaccharides (LPS) derived from the outer membrane of gram-negative bacteria increase plasma concentrations of proinflammatory cytokines, such as IL-6 and TNF-α and disinhibit the production of anti-inflammatory cytokines leading to negative affects along with sympathoadrenal and HPA axis’s stimulation (Dickson and Lehmann, 2019; Godin et al., 1996; Grigoleit et al., 2011; van der Poll et al., 1996b). Escherichia coli a gram-negative bacteria, elicits increases in plasma IL-6, IL-8 and TNF-α, IL-1ra, and cortisol (Godin et al., 1996; Michie et al., 1988; Moller et al., 2002; Suffredini et al., 1999). Taken together, these validity aspects point to infection as a reasonable naturalistic model for studying stress in the context of respective infectious syndromes.
Hypovolemia.
Water constitutes about 60% of body weight in men and 50% in women; its content balance is maintained by tightly synchronized homeostatic mechanisms (Melendez Rivera and Anjum, 2023). Such balance may be threatened by kidney-related and -unrelated pathophysiological processes (Jameson J, 2018). The former involves compromised osmosis-mediated reabsorption of sodium and water along the kidney tubules with the resultant reduction in the intravascular water content (Stremple et al., 1966). Hemorrhage is the most common cause of the kidney unrelated hypovolemia (Lowe, 1990; Sawka, 1992). Clinical manifestations of both types of hypovolemia are of questionable stress construct validity (reasonable convergent validity) and include anxiety, agitation, sweating, dizziness, weakness, fatigue, confusion and tachycardia (Sawka et al., 2015).
The neuroendocrine response pattern to hypovolemia is biphasic. At the initial stage, drops in the circulating volume are detected via the cardiac and arterial baroreceptors that activate the sympathoneural- and RAAS systems (Schadt and Ludbrook, 1991) in an attempt to maintain normal cardiac output and blood pressure by redistributing the flow to the vital organs amidst peripheral vasoconstriction and consequent hypoperfusion. Not so, when blood pressure falls below the homeostatic range at about 30% loss of volume (Goldstein, 1995). Such an event triggers an opposite response pattern viz., inhibition of the sympathoneural system and predominant effect of the epinephrine secretion from the adrenals, which leads to vasodilatation and further drop in blood pressure, that is to say, an unstable positive feedback loop with progressive worsening of the clinical condition (Goldstein, 1995). The volume changes are also detected by the important homeostat located in the PVN and supraoptic nuclei that produce and release vasopressin into the peripheral circulation from the neurohypophysis where it is stored. Thus, urinary output is diminished while the activity of the renin-angiotensin-aldosterone system is augmented. All-in-all hypovolemia represents a state wherein a non-specific event leads to a specific stress response i.e., the activation of the sympathoneural system (discriminant validity). The initially specific response becomes more generalized as blood pressure decreases and clinical condition continues to deteriorate. And so, with hypovolemia may not be a universal stress model given the dynamic nature of the criterion- and discriminatory validity.
Glucoprivation.
In humans (and in other primates), glucose is the major energy source for the central nervous system (CNS). The specific nature of the primary glucostat governing compensatory responses to declining glucose levels is still being debated with potential roles in the maintenance of blood glucose concentrations within the tight physiological range attributed to the hypothalamus, liver, endocrine pancreas, and pancreatic islets of Langerhans themselves (Briski et al., 2021; Raben et al., 1996; Rodriguez-Diaz et al., 2018). All are a part of a complex network of interrelated peripheral and central structures, each of which exhibits a unique function within the context of glucoregulation via insulin and counterregulatory hormones such as glucagon, growth hormone, AVP and prolactin (Elman et al., 2006).
Because glucose is neither stored nor produced by the CNS, there is a need to reinforce and reward behaviors aimed at the procurement of this fuel which is indispensable for survival (Elman et al., 2006). Thus, glucose and other sweet tasting saccharides have evolved as a primary reward. This type of reward is unconditioned on prior learning (Wise, 2002) so that newborns, consistently with the taste of the ultimate item on their menu, mother’s milk, display a strong preference for sweetness (Steiner et al., 2001). Glucose consumption indeed produces immediate pleasurable affective states (Wise et al., 2016) mediated through μ-opiate neurotransmission within the scattered network of subcortical and brainstem nuclei (Elman et al., 2006). Opioids enhance sugar pleasantness (Elman et al., 2020), whereas sugar consumption increases endogenous opiates release (i.e., feedforward interaction) so that stress reduction (Tryon et al., 2015) and even analgesia (Mercer and Holder, 1997; Segato et al., 1997) may ensue.
Glucose deprivation is conversely a formidable homeostatic challenge (Gibbons et al., 2012) manifested in the constructs of marked anxiety and distress (Breier, 1989; Elman and Breier, 1997; Elman et al., 2004) measured with self-ratings (Adler et al., 2000; Elman et al., 2004). The global nature of the threat posed by neuroglucopenia necessities a whole-body level hormonal response. Hence is the recruitment of the sympathoadrenal system (Elman et al., 2004) and the HPA axis (Elman et al., 1998; Kerr et al., 1989) with outpouring of epinephrine (Elman et al., 2001b; Scheurink and Ritter, 1993) that precedes sympathetic activation and may be apparent below subjective perception threshold of hypoglycemia (Schwartz et al., 1987) as well as the RAAS (Lowder et al., 1976) and AVP (Elman et al., 2003). Furthermore, glucoprivation is linked to increased proinflammatory cytokines (IL-6, IL-8, TNF-α and IL-1β), markers of lipid peroxidation and leukocytosis (Razavi Nematollahi et al., 2009). Such pattern along with a subjective sense of hunger (Elman et al., 1998; Elman et al., 2003) is a persuasive example for the generalization of stress responses (convergent validity) c.f., early hypovolemia characterized by a relatively selective recruitment of the sympathetic nervous system entrusted with the redistribution of the blood flow to vital organs (Goldstein and Kopin, 2008).
Insulin is the primary glucoprivic agent, but its use may be confounded by only short-lived and rapidly counter-regulated (predominately by glucagon) hypoglycemia (Elman et al., 2004). Use of pharmacological doses of a non-metabolizable glucose analog, 2-deoxyglucose (2DG), is an alternative technique resulting in transient disruption of glycolysis and interference with cellular utilization of glucose (Horton et al., 1973). The value of using 2-DG is that it eliminates confounds of insulin’s direct effects and produces centrally generated clinical state similar to hypoglycemia even though it elevates plasma glucose levels, which is well tolerated (Elman and Breier, 1997; Takahashi et al., 1994)—features adding to discriminant validity.
Heat.
Heat or exposure to high temperatures is a renowned stress model, amendable to subjective assessments such as questionnaires measuring heat stress thresholds and factors contributing to heat stress in urban contexts (Beckmann, 2021) coinciding with the construct validity of psychological stress. Heat stress activates the sympathoadrenal system (Boonruksa et al., 2020; Brenner et al., 1998; Cheshire, 2016; Cramer and Jay, 2016; Yamamoto et al., 2007) causing the release of norepinephrine and epinephrine (McMorris et al., 2006; Salim Al-Hadramy, 1989). The HPA axis (Brenner et al., 1998; Follenius et al., 1982; Wang et al., 2015) activation by heat together with the RAAS- (Kosunen et al., 1976) and AVP (Montain et al., 1997; Yang and Gordon, 2002) systems determine the criterion validity of the heat model. Also, while acute heat exposure may have anti-inflammatory effects (Ganesan et al., 2016), chronic heat exposure suppresses proper immune activity (Cantet et al., 2021) with corresponding alterations in the cytokine concentration (Nagai, 2001).
As to convergent validity, heat stress shares some similarities with models involving heat-induced dehydration (Davies et al., 2023) or painful stimuli, such as the cold pressor (Velasco et al., 1997) or heat-induced pain (Elman et al., 2018). High temperatures also lead to negative affective states e.g., irritability, fatigue, anger, aggression (Anderson, 2000) and reduced cognitive performance (Mazloumi et al., 2014). Discriminant validity can be established by distinguishing heat-induced stress from conditions like heat exhaustion (Gauer and Meyers, 2019), where the stress response has progressed to a pathological state. Delineating these differences is crucial in differentiating adaptive stress responses from the maladaptive ones.
Sleep Deprivation.
The primary sleep function is postulated to involve optimization of energy utilization by reallocating energy resources to vital biological functions like cellular maintenance and growth, immune response, and neural plasticity, as opposed to wake-related activities such as alertness, searching for food, and reproduction (Schmidt, 2014). Sleep deprivation stress model (McEwen and Karatsoreos, 2015) centers on a period of acute sleep loss, typically spanning 24 to 72 hours (Alhola and Polo-Kantola, 2007) and it conforms to various constructs of perceived stress (Gardani et al., 2022), including global measures of perceived stress (Cohen et al., 1983) and specific tools like the Depression Anxiety Stress Subscale (Lovibond and Lovibond, 1995), Undergraduate Stress Questionnaire (Lovibond and Lovibond, 1995) and the stress component of the Patient Health Questionnaire (Schlarb et al., 2017).
Sleep deprivation leads to inconsistent findings in the sympathoadrenal system criterion. Some studies report increases in the activity (Lusardi et al., 1999; Tochikubo et al., 1996), while others show no significant changes (Kato et al., 2000; Martin and Chen, 1984). These inconsistent findings may be partially explained by the complexity of the stressor as it pertains to individual differences, variations in sleep deprivation severity and duration, timing of measurements, adaptation, and interactions with other variables. However, the HPA axis’ activation patterns during sleep deprivation seem to be consistent (Hirotsu et al., 2015; Nollet et al., 2020; Schmidt, 2014; Tsai et al., 1989). Conversely, sleep deprivation causes a drop in RAAS and AVP activity (Kamperis et al., 2010; Kunzel et al., 2020; Mahler et al., 2012). Moreover, sleep deprivation heightens the levels of pro-inflammatory cytokines e.g., IL-6, TNFα and C-reactive protein (Patel et al., 2009). Lack of sleep renders the host susceptible to infections evident in the link between sleep deprivation and an altered immune system's ability to respond effectively to pathogens, vaccines, or other immune challenges (Ibarra-Coronado et al., 2015). Chronic inflammation resulting from poor sleep is linked to various health issues (convergent validity), including cardiovascular diseases, diabetes, autoimmune disorders (Ibarra-Coronado et al., 2015), depression, and anxiety (Ben Simon et al., 2020; McEwen and Karatsoreos, 2015). Sleep deprivation specifically leads to diminished alertness, excessive sleepiness, and risk of accidents (Colten et al., 2006) effects that help differentiate it from other stress models or conditions (discriminant validity).
Pain remains a global public health challenge, afflicting 1.5 billion people worldwide (Galea, 2017) and ranking as the most prevalent concern reported to healthcare providers (Crook et al., 1984). Being critical for the survival, pain system is embedded within the overlapping (Elman and Borsook, 2019; Elman et al., 2009; Elman et al., 2018) reward and stress circuitry (Insel, 2003) responsible for continued existence of individuals and species via pursuit of food, water, sex, and social affiliations (Elman et al., 2011), an idea consistent with the reward-pain continuum (Borsook et al., 2007). An important offshoot of this idea is that pain serves as a functional probe of the brain reward and stress systems by allowing to measure the contribution of pain and analgesia to the function of the respective neurobiological systems and to identify a clinically distinct subgroup of patients for whom potentiation of reward function and/or stress reduction interventions may be beneficial (Elman and Borsook, 2016; Elman et al., 2013; Elman et al., 2011). As a stress model, pain has demonstrated solid construct validity when assessed through various stress questionnaires (Wippert et al., 2022), such as Cohen's Perceived Stress Scale (Vinstrup et al., 2020)
Criterion validity demonstrated through the involvement of neuroendocrine stress pathways is inconsistent (Janssen et al., 1998). The initial sympathoadrenal analgesic effect, mediated by the locus coeruleus, is followed by its enhancements of pain perception (Taylor and Westlund, 2017; Tsigos et al., 1993). Pain-induced cortisol release (Tennant, 2013) exerts a similar early pain reliving effect while sensitizing chronic pain responses (Griep et al., 1998; Hannibal and Bishop, 2014; Tennant and Hermann, 2002) that akin to PTSD (Elman et al., 2013) may lead to an eventual hypocorticolism (McEwen and Kalia, 2010; Muhtz et al., 2013). The angiotensin effects are likewise both algesic and analgesic (Acosta, 2023). The AVP impacts are usually analgesic (Mavani et al., 2015; Mogil et al., 2011). Thence, pain has limited neuroendocrine criterion validity.
Pain definition by the International Association for the Study of Pain (IASP) as “a personal experience that is influenced to varying degrees by biological, psychological, and social factors” (IASP, 2020) echoes the neuroanatomical overlap of “pain-related emotions’’ and ‘‘emotional pain’’ (Elman and Borsook, 2016) and the convergence of pain experience of either physical and/or psychological etiology (Elman et al., 2013; Elman et al., 2011) including the heightened comorbidity of pain with depressive and anxiety disorders and PTSD (Borsook et al., 2016; Elman et al., 2013; Elman et al., 2011; Kim et al., 2022). Besides, due to the essential features e.g., persistent relieving of stress, responding with hopelessness, catastrophizing or horror and avoidance of pain-related situations, chronic pain may be even construed as a form of PTSD (Elman et al., 2013). The discriminant validity is apparent from the involvement of the neural sensory apparatus (Elman and Borsook, 2016). Albeit “cannot be inferred solely from activity in sensory neurons” (IASP, 2020), pain frequently activates sensory pathways associated with detecting and processing painful stimuli. Additionally, the concept of pain-induced pleasure or algophilia (Elman and Borsook, 2016) may deter from the distinct nature of pain as a stressor, separate from other forms of psychological or physical stress.
11.4. Pharmacological Stressors
Adenosine, a nucleoside composed of adenine and ribose sugar, is a key component of the adenosine triphosphate molecule (Dunn and Grider, 2023). As such, adenosine is involved in storing and transferring biochemical energy and so fueling numerous biochemical processes within cells (Dunn and Grider, 2023). Adenosine receptors are widely distributed throughout the body (Menezes, 2017) and implicated in mood and anxiety disorders (van Calker et al., 2019). Adenosine is used for the induction of pharmacological stress in myocardial perfusion imaging studies (Polad and Wilson, 2002). When administered intravenously it causes a brief tachycardia and hypotension due to vasodilation (Guieu et al., 2020); cardiovascular changes may partially mimic some of the physiological responses during stress (i.e., questionable construct and convergent validities). Adenosine test has no criterion validity as it suppresses the sympathoadrenal (Richardt et al., 1989) and HPA axis’ (Charney et al., 1985) activity. On the other hand, adenosine causes the release of renin (Taddei et al., 1992). Adenosine's unique characteristics, like transient AV block (Keim et al., 1992; O'Keefe et al., 1992) and promoting coronary steal (Aetesam-Ur-Rahman et al., 2021) while increasing cardiac perfusion (Yimcharoen et al., 2020), set it apart from traditional stressors. The distinctive features can serve as discriminant validity indicators, as they differ from other stress models and emphasize its suitability for cardiac stress testing.
Dobutamine is a selective beta-1 adrenergic receptor agonist is uniquely suited to increase cardiac contractility (Bylund et al., 2008; DrugBank, 2023). Other than treatment of heart failure, dobutamine (Ashkar et al., 2023) is employed as an alternative to exercise electrocardiography for diagnosing coronary artery disease (Gonzalez and Beller, 2017). This methodology has not yet been systematically assessed for construct validity by juxtaposing physiological responses it induces to self-reported physical and mental stress as it may be experienced in daily life (Wagner et al., 1996). The criterion validity is partial as dobutamine infusion leads to declines in sympathoadrenal (Velez-Roa et al., 2003) activity and increases in cortisol (Seo et al., 2003). Dobutamine converge with adenosine (Polad and Wilson, 2002) as a cardiac stressor along with co-occurring chest pain, breathlessness, or dizziness (SVHC, 2023). Discriminant validity is demonstrated by the dual roles in the treatment of heart failure and in cardiac evaluation.
Yohimbine. Yohimbine is a pharmacological agent, produced from a tree (pausinystalia yohimbe) bark, that induces mental and physical stress (See and Waters, 2010). It is a prototypical alpha-2 adrenoceptor antagonist even though it also influences other receptors, D2, alpha-1 and 5HT1a (Chemical, 2023). Yohimbine construct validity as a stress model (See and Waters, 2010) is demonstrated via self-reported stress ratings (Elman et al., 2012) and psychometric assessments (Umhau et al., 2011) in individuals who receive the drug. Yohimbine's effects on the sympathoadrenal system (Anadón, 2016) and HPA axis, but not AVP (Peskind et al., 1989), as evidenced by increased blood pressure, heart rate, plasma norepinephrine levels (Biaggioni et al., 1994; Petrie et al., 2000; Swann et al., 2013; Tanaka et al., 2000) and cortisol (Fricke et al., 2023; Grunhaus et al., 1989; Price et al., 1986) concentrations, establish its criterion validity as a stress model. In preclinical models yohimbine also increased plasma renin (Pfister and Keeton, 1988) and inhibited IL-6, TNF-α and IL-1β overproduction. Yohimbine evokes a variety of various negative affective states, including anxiety, panic, restlessness, and agitation (Cimolai and Cimolai, 2011; Holmberg and Gershon, 1961; Morris et al., 2020) and even manic symptoms (Price et al., 1984) thereby demonstrating convergent validity. While yohimbine is primarily known for inducing stress-like responses, its FDA-approved use in the treatment of erectile dysfunction (Morales, 2000), thus alleviating a lot of stress, raises questions about its specificity as a stress model and suggests some ambiguity regarding discriminant validity.
12. Conclusions
Leonard Bernstein reckoned that to “achieve great things, two things are needed; a plan, and not quite enough time [i.e., stress],” whereas, according to Hans Selye, “It’s not stress that kills us, it’s our reaction to it.” As such, it is understandable that stress and its models cannot be viewed as ‘one size fits all.’ This review compares the outcomes of experimental stress models to underscore the breadth of the stress phenomena where some features are shared while other features may not generalize. For instance, the HPA axis seems to be engaged by most stressors whereas there are no parallels in the psychosocial stress literature for the counterregulatory response typical of glucopirvation (Elman et al., 1998; Elman et al., 2004; Kim et al., 2021). The recognition that various stress models may share a common neurobiological foundation could have important mechanistic and therapeutic implications.
For instance, the sympathoadrenal system and the HPA axis effects sensitize over time (Franco et al., 2016; Gresch et al., 1994; Jedema et al., 1999) and so cross-sensitization might occur, that is, early life traumatization (Belda et al., 2016) increase susceptibility to the development of mental and physical morbidity e.g., depression, anxiety, hypertension (Stein et al., 2010) and other types of cardiovascular disease (Bandoli et al., 2017) particularly in the context of unrelated stress exposure (Stein et al., 2010). Consequently, identifying cross-sensitization mechanisms may be important for the primary and secondary prevention of such morbidity. If vulnerability factors could be identified, they might be used to screen people at risk for the development of stress-related disorders. Those found to possess high vulnerability might be counseled to avoid stress (primary prevention) or targeted for early intervention even in the presence of mild stress-related dysregulation (secondary prevention).
Remarkable advances of stress research have shed light on the fundamental mechanisms at the cellular-, molecular- (epi)genetic-, neurocircuitry- and whole person levels (Feder et al., 2009; McEwen and Akil, 2020) as well as refuted the stress non-specificity doctrine (Goldstein, 1995). The conclusiveness of many studies is, however, tarnished by the lack of a uniform nosology contributing to the ongoing debate about scientific veracity of the stress concept. Here we proposed a set of validity criteria charting a path forward for a more focused and consensus-driven approach. At the same time our findings point to an inability of any stress model to reflect on the full nature of the stressful experiences. Depending on the circumstances, one model may offer greater heuristic value than another; at times several models may be essential whereas there may be also situations wherein no model can accurately depict the unique human perceptions/experiences and their emotional, neurochemical, and behavioral manifestations. The latter may the case for some of the emotional aspects of trauma. Yet, perturbations of the HPA axis are valuable in the trauma aftermath in order to define the glucocorticoid receptor hypersensitivity typical of the PTSD syndrome (Somvanshi et al., 2020). Concurrent elucidation of the sympathetic system hyperactivity criterion (Forstenpointner et al., 2022) is instrumental for tailoring the anti-adrenergic therapeutic regiments in order to block the traumatic memories reconsolidation (Brunet et al., 2018). Likewise, challenges of the glucoregulatory system via glucoprivic procedures may point to the ways of counteracting metabolic derailments prevalent in PTSD patients (Palmer et al., 2021).
This line of thought could lead to the integration of stress models into routine clinical practice aimed at individualization of the preventive, diagnostic and therapeutic interventions. The most serious challenge is, of course, the choice of relevant tests, the assertion of their pertinent validities and generation of hypotheses that may be constructively tested in real life patients. After all, patients’ wellbeing is not entirely amendable to straightforward definitions. Hence is why it is important to determine the ultimate targets for the employed stress models e.g., environmental, lifestyle, psychological neuroendocrine, etc... Conversely, a choice of an invalid procedure could be counterproductive leading to inconclusive or plainly irrelevant results.
As with identification of the proper procedure, exigencies of patients’ characterization (e.g., comorbidities, medications, demographics, personality features and health status) could also confound the outcomes of stress studies. For example, the presence of depression even long time prior to the study may alter the brain’s stress system (Admon et al., 2015), as might the concurrent use of psychotropic medicines (Elman and Borsook, 2019; Elman et al., 2018). Given the substantial prevalence of depression (Group, 1992), however, implementing this as an exclusion factor would likely rule out such a high percentage of patient candidates as to make clinical use of stress studies unfeasible. Although we recognize that some disagreements may arise regarding the exclusion decisions, reasonable compromises could be certainly made between diagnostic pureness and feasibility in order to balance meticulousness with pragmatics by excluding/adjusting for acute mental and physical conditions as far as possible.
Personalized medicine is probably the optimally suited setting for matching the proper tests to the clinically eligible patients. Provided that the main objective of personalized medicine is to refine and improve preventive, diagnostic and therapeutic interventions, exposition of individuals’ acute stress responses and longer-term adjustments to challenging perturbations may be an important intermediary in this process. An ensuing medical field focused on antecedents of stress responses (i.e., resilience factors), the responses themselves and consequences of stress exposure could propel a unique multidisciplinary collaborative forum unrestricted by unnecessary boundaries separating medical specialties among each other and from complementary clinical disciplines (e.g., medicine, psychology, nursing, social work and physical therapy) thus contributing to greater depth and sophistication of clinical formulations and therapeutic plans (Elman et al., 2011). The time seems to be ripe to engage in such an endeavor at least from the scientific and clinical standpoints.
Highlights.
Stress is a multidimensional concept.
Examination of distinct models based on the stressor type aids stress comprehension.
Mental and physical stress models show reasonable construct- and criterion validity assessed via psychometric ratings and physiological responses.
The construct- and criterion validity findings are less robust for pharmacological perturbations.
Convergent and discriminant validity vary depending on the nature of the stressor stimulus.
Personalizing stress model selection based on stressor traits enhances relevance for diagnosis, prevention, and treatment but requires further refinement.
Acknowledgment
The authors gratefully acknowledge anonymous reviewers for their insightful and constructive comments on the manuscript.
Support:
RF and DB received support from NIH R01NS105844
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acosta CG, Patterson SI, Valdez SR, Seltzer AM, 2023. Chapter 23 - Angiotensin and pain. Academic Press. [Google Scholar]
- Adams TB, Wharton CM, Quilter L, Hirsch T, 2008. The association between mental health and acute infectious illness among a national sample of 18- to 24-year-old college students. J Am Coll Health 56, 657–663. [DOI] [PubMed] [Google Scholar]
- Adler CM, Elman I, Weisenfeld N, Kestler L, Pickar D, Breier A, 2000. Effects of acute metabolic stress on striatal dopamine release in healthy volunteers. Neuropsychopharmacology 22, 545–550. [DOI] [PubMed] [Google Scholar]
- Admon R, Holsen LM, Aizley H, Remington A, Whitfield-Gabrieli S, Goldstein JM, Pizzagalli DA, 2015. Striatal Hypersensitivity During Stress in Remitted Individuals with Recurrent Depression. Biol Psychiatry 78, 67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aetesam-Ur-Rahman M, Brown AJ, Jaworski C, Giblett JP, Zhao TX, Braganza DM, Clarke SC, Agrawal BSK, Bennett MR, West NEJ, Hoole SP, 2021. Adenosine-Induced Coronary Steal Is Observed in Patients Presenting With ST-Segment-Elevation Myocardial Infarction. J Am Heart Assoc 10, e019899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilo Mir S, Garcia Pages E, Lopez Barbeito B, Ribeiro TC, Garzon-Rey JM, Aguilo Llobet J, 2021. Design and validation of an electrophysiological based tool to assess chronic stress. Case study: burnout syndrome in caregivers. Stress 24, 384–393. [DOI] [PubMed] [Google Scholar]
- Akil H, Nestler EJ, 2023. The neurobiology of stress: Vulnerability, resilience, and major depression. Proc Natl Acad Sci U S A 120, e2312662120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alanazi MM, Havranek T, Bakos J, Cubeddu LX, Castejon AM, 2020. Cell proliferation and anti-oxidant effects of oxytocin and oxytocin receptors: role of extracellular signal-regulating kinase in astrocyte-like cells. Endocr Regul 54, 172–182. [DOI] [PubMed] [Google Scholar]
- Alhola P, Polo-Kantola P, 2007. Sleep deprivation: Impact on cognitive performance. Neuropsychiatr Dis Treat 3, 553–567. [PMC free article] [PubMed] [Google Scholar]
- Allen AP, Kennedy PJ, Cryan JF, Dinan TG, Clarke G, 2014. Biological and psychological markers of stress in humans: focus on the Trier Social Stress Test. Neurosci Biobehav Rev 38, 94–124. [DOI] [PubMed] [Google Scholar]
- Allen AP, Kennedy PJ, Dockray S, Cryan JF, Dinan TG, Clarke G, 2017. The Trier Social Stress Test: Principles and practice. Neurobiol Stress 6, 113–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen MJ, Sharma S, 2023. Physiology, Adrenocorticotropic Hormone (ACTH), StatPearls, Treasure Island (FL). [PubMed] [Google Scholar]
- American Psychiatric A, 2022. Diagnostic and statistical manual of mental disorders : DSM-5- TR : text revision, 5th edition, text revision ed. American Psychiatric Association Publishing, Washington, DC. [Google Scholar]
- Ames MK, Atkins CE, Pitt B, 2019. The renin-angiotensin-aldosterone system and its suppression. J Vet Intern Med 33, 363–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin MN, Siddiqui SA, Ibrahim M, Hakim ML, Ahammed MS, Kabir A, Sultana F, 2020. Inflammatory cytokines in the pathogenesis of cardiovascular disease and cancer. SAGE Open Med 8, 2050312120965752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amirkhan JH, 2018. A Brief Stress Diagnostic Tool: The Short Stress Overload Scale. Assessment 25, 1001–1013. [DOI] [PubMed] [Google Scholar]
- Amirkhan JH, Urizar GG, Clark S, 2015. Criterion validation of a stress measure: the Stress Overload Scale. Psychol Assess 27, 985–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anadón AM-L, M.R., Ares I, Martínez MA, 2016. Chapter 60 - Interactions between Nutraceuticals/Nutrients and Therapeutic Drugs. Academic Press. [Google Scholar]
- Anderson CA, Anderson KB, Dorr N, DeNeve KM, Flanagan M, 2000. Temperature and aggression. Academic Press. [Google Scholar]
- Andrade C, 2018. Internal, External, and Ecological Validity in Research Design, Conduct, and Evaluation. Indian J Psychol Med 40, 498–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreis PG, Neri G, Meneghelli V, Mazzocchi G, Nussdorfer GG, 1992. Effects of interleukin-1 beta on the renin-angiotensin-aldosterone system in rats. Res Exp Med (Berl) 192, 1–6. [DOI] [PubMed] [Google Scholar]
- Anisman H, Merali Z, 1999. Understanding stress: characteristics and caveats. Alcohol Res Health 23, 241–249. [PMC free article] [PubMed] [Google Scholar]
- Arguinchona JH, Tadi P, 2023. Neuroanatomy, Reticular Activating System, StatPearls, Treasure Island (FL). [PubMed] [Google Scholar]
- Armando I, Villar VA, Jose PA, 2011. Dopamine and renal function and blood pressure regulation. Compr Physiol 1, 1075–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashkar H, Adnan G, Makaryus AN, 2023. Dobutamine, StatPearls, Treasure Island (FL). [PubMed] [Google Scholar]
- Assaf AM, Al-Abbassi R, Al-Binni M, 2017. Academic stress-induced changes in Th1- and Th2-cytokine response. Saudi Pharm J 25, 1237–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atlas SA, Case DB, Sealey JE, Laragh JH, 1981. Relationship between plasma renin and cortisol in hypertensive patients. Clin Sci (Lond) 61 Suppl 7, 265s–268s. [DOI] [PubMed] [Google Scholar]
- Back SE, Acierno R, Saraiya TC, Harley B, Wangelin B, Jarnecke AM, McTeague LM, Brown DG, Ana ES, Rothbaum AO, Adams RJ, 2022. Enhancing Prolonged Exposure therapy for PTSD using physiological biomarker-driven technology. Contemp Clin Trials Commun 28, 100940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker C, Richards LJ, Dayan CM, Jessop DS, 2003. Corticotropin-releasing hormone immunoreactivity in human T and B cells and macrophages: colocalization with arginine vasopressin. J Neuroendocrinol 15, 1070–1074. [DOI] [PubMed] [Google Scholar]
- Bali A, Jaggi AS, 2013. Angiotensin as stress mediator: role of its receptor and interrelationships among other stress mediators and receptors. Pharmacol Res 76, 49–57. [DOI] [PubMed] [Google Scholar]
- Bali A, Jaggi AS, 2015. Clinical experimental stress studies: methods and assessment. Rev Neurosci 26, 555–579. [DOI] [PubMed] [Google Scholar]
- Bamert M, Inauen J, 2022. Physiological stress reactivity and recovery: Some laboratory results transfer to daily life. Front Psychol 13, 943065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandoli G, Campbell-Sills L, Kessler RC, Heeringa SG, Nock MK, Rosellini AJ, Sampson NA, Schoenbaum M, Ursano RJ, Stein MB, 2017. Childhood adversity, adult stress, and the risk of major depression or generalized anxiety disorder in US soldiers: a test of the stress sensitization hypothesis. Psychol Med 47, 2379–2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baribeau DA, Anagnostou E, 2015. Oxytocin and vasopressin: linking pituitary neuropeptides and their receptors to social neurocircuits. Front Neurosci 9, 335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bataillard A, del Rey A, Klusman I, Arditi GM, Besedovsky HO, 1992. Interleukin-1 stimulates aldosterone secretion: involvement of renin, ACTH, and prostaglandins. Am J Physiol 263, R840–844. [DOI] [PubMed] [Google Scholar]
- Bauerly KR, Jones RM, Miller C, 2019. Effects of Social Stress on Autonomic, Behavioral, and Acoustic Parameters in Adults Who Stutter. J Speech Lang Hear Res 62, 2185–2202. [DOI] [PubMed] [Google Scholar]
- Beckmann SK, Hiete M, Beck C, 2021. Threshold temperatures for subjective heat stress in urban apartments—Analysing nocturnal bedroom temperatures during a heat wave in Germany.
- Beerda B, Schilder MB, Janssen NS, Mol JA, 1996. The use of saliva cortisol, urinary cortisol, and catecholamine measurements for a noninvasive assessment of stress responses in dogs. Horm Behav 30, 272–279. [DOI] [PubMed] [Google Scholar]
- Belda X, Nadal R, Armario A, 2016. Critical features of acute stress-induced cross-sensitization identified through the hypothalamic-pituitary-adrenal axis output. Sci Rep 6, 31244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell C, Johnston D, Allan J, Pollard B, Johnston M, 2017. What do Demand-Control and Effort-Reward work stress questionnaires really measure? A discriminant content validity study of relevance and representativeness of measures. Br J Health Psychol 22, 295–329. [DOI] [PubMed] [Google Scholar]
- Ben Simon E, Vallat R, Barnes CM, Walker MP, 2020. Sleep Loss and the Socio-Emotional Brain. Trends Cogn Sci 24, 435–450. [DOI] [PubMed] [Google Scholar]
- Benigni A, Cassis P, Remuzzi G, 2010. Angiotensin II revisited: new roles in inflammation, immunology and aging. EMBO Mol Med 2, 247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berecek KH, Olpe HR, Jones RS, Hofbauer KG, 1984. Microinjection of vasopressin into the locus coeruleus of conscious rats. Am J Physiol 247, H675–681. [DOI] [PubMed] [Google Scholar]
- Beresford T, Wahlberg L, Hipp D, Ronan PJ, 2021. Psychological Adaptive Mechanism Maturity Predicts Good Outcomes in Treatment for Refractory PTSD. Front Psychol 12, 718451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD, 2003. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev 42, 33–84. [DOI] [PubMed] [Google Scholar]
- Berton O, Durand M, Aguerre S, Mormede P, Chaouloff F, 1999. Behavioral, neuroendocrine and serotonergic consequences of single social defeat and repeated fluoxetine pretreatment in the Lewis rat strain. Neuroscience 92, 327–341. [DOI] [PubMed] [Google Scholar]
- Bethin KE, Vogt SK, Muglia LJ, 2000. Interleukin-6 is an essential, corticotropin-releasing hormone-independent stimulator of the adrenal axis during immune system activation. Proc Natl Acad Sci U S A 97, 9317–9322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biaggioni I, Robertson RM, Robertson D, 1994. Manipulation of norepinephrine metabolism with yohimbine in the treatment of autonomic failure. J Clin Pharmacol 34, 418–423. [DOI] [PubMed] [Google Scholar]
- Bjorkqvist K, 2001. Social defeat as a stressor in humans. Physiol Behav 73, 435–442. [DOI] [PubMed] [Google Scholar]
- Bjorntorp P, Holm G, Rosmond R, Folkow B, 2000. Hypertension and the metabolic syndrome: closely related central origin? Blood Press 9, 71–82. [DOI] [PubMed] [Google Scholar]
- Blevins CA, Weathers FW, Davis MT, Witte TK, Domino JL, 2015. The Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5): Development and Initial Psychometric Evaluation. J Trauma Stress 28, 489–498. [DOI] [PubMed] [Google Scholar]
- Blum K, Dennen CA, Elman I, Bowirrat A, Thanos PK, Badgaiyan RD, Downs BW, Bagchi D, Baron D, Braverman ER, Gupta A, Green R, McLaughlin T, Barh D, Gold MS, 2022. Should Reward Deficiency Syndrome (RDS) Be Considered an Umbrella Disorder for Mental Illness and Associated Genetic and Epigenetic Induced Dysregulation of Brain Reward Circuitry? J Pers Med 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocchiaro P, Zimbardo P, 2017. On the dynamics of disobedience: experimental investigations of defying unjust authority. Psychol Res Behav Manag 10, 219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond RF, Johnson G 3rd, 1985. Vascular adrenergic interactions during hemorrhagic shock. Fed Proc 44, 281–289. [PubMed] [Google Scholar]
- Boonruksa P, Maturachon T, Kongtip P, Woskie S, 2020. Heat Stress, Physiological Response, and Heat-Related Symptoms among Thai Sugarcane Workers. Int J Environ Res Public Health 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsook D, Becerra L, Carlezon WA Jr., Shaw M, Renshaw P, Elman I, Levine J, 2007. Reward-aversion circuitry in analgesia and pain: implications for psychiatric disorders. Eur J Pain 11, 7–20. [DOI] [PubMed] [Google Scholar]
- Borsook D, Hargreaves R, Becerra L, 2011. Can Functional Magnetic Resonance Imaging Improve Success Rates in CNS Drug Discovery? Expert Opin Drug Discov 6, 597–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsook D, Linnman C, Faria V, Strassman AM, Becerra L, Elman I, 2016. Reward deficiency and anti-reward in pain chronification. Neurosci Biobehav Rev 68, 282–297. [DOI] [PubMed] [Google Scholar]
- Bowirrat A, Elman I, Dennen CA, Gondré-Lewis MC, Cadet JL, Khalsa J, Baron D,Soni D, Gold MS, McLaughlin TJ, Bagchi D, Braverman ER, Ceccanti M, Thanos PK, Modestino EJ, Sunder K, Jafari N, Zeine F, Badgaiyan RD, Barh D, Makale M, Murphy KT, Blum K. Neurogenetics and Epigenetics of Loneliness. Psychol Res Behav Manag. 2023 [DOI] [PMC free article] [PubMed]
- Boyd JH, Holmes CL, Wang Y, Roberts H, Walley KR, 2008. Vasopressin decreases sepsis-induced pulmonary inflammation through the V2R. Resuscitation 79, 325–331. [DOI] [PubMed] [Google Scholar]
- Bracken MB, 2009. Why animal studies are often poor predictors of human reactions to exposure. J R Soc Med 102, 120–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breier A, 1989. A.E. Bennett award paper. Experimental approaches to human stress research: assessment of neurobiological mechanisms of stress in volunteers and psychiatric patients. Biol Psychiatry 26, 438–462. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Southwick SM, Charney DS, 1996. Noradrenergic mechanisms in stress and anxiety: II. Clinical studies. Synapse 23, 39–51. [DOI] [PubMed] [Google Scholar]
- Brenner I, Shek PN, Zamecnik J, Shephard RJ, 1998. Stress hormones and the immunological responses to heat and exercise. Int J Sports Med 19, 130–143. [DOI] [PubMed] [Google Scholar]
- Brindley DN, 1992. Neuroendocrine regulation and obesity. Int J Obes Relat Metab Disord 16 Suppl 3, S73–79. [PubMed] [Google Scholar]
- Briski KP, Ibrahim MMH, Mahmood A, Alshamrani AA, 2021. Norepinephrine Regulation of Ventromedial Hypothalamic Nucleus Astrocyte Glycogen Metabolism. Int J Mol Sci 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromet E, Florescu S, Girolamo G, Navarro-Mateu F, & Karam E, 2018. Natural and Human-Made Disasters. Cambridge University Press, Cambridge. [Google Scholar]
- Brouillard C, Carrive P, Camus F, Benoliel JJ, Sevoz-Couche C, 2019. Vulnerability to stress consequences induced by repeated social defeat in rats: Contribution of the angiotensin II type 1 receptor in cardiovascular alterations associated to low brain derived neurotrophic factor. Eur J Pharmacol 861, 172595. [DOI] [PubMed] [Google Scholar]
- Brouwer AM, Hogervorst MA, 2014. A new paradigm to induce mental stress: the Sing-a- Song Stress Test (SSST). Front Neurosci 8, 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet A, Saumier D, Liu A, Streiner DL, Tremblay J, Pitman RK, 2018. Reduction of PTSD Symptoms With Pre-Reactivation Propranolol Therapy: A Randomized Controlled Trial. Am J Psychiatry 175, 427–433. [DOI] [PubMed] [Google Scholar]
- Bruno RM, Ghiadoni L, Seravalle G, Dell'oro R, Taddei S, Grassi G, 2012. Sympathetic regulation of vascular function in health and disease. Front Physiol 3, 284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buemann B, Marazziti D, Uvnas-Moberg K, 2021. Can intravenous oxytocin infusion counteract hyperinflammation in COVID-19 infected patients? World J Biol Psychiatry 22, 387–398. [DOI] [PubMed] [Google Scholar]
- Bylund DB, Enna SJ, Elsevier S, 2008. xPharm : the comprehensive pharmacology reference. Elsevier, Place of publication not identified. [Google Scholar]
- Campioni MR, Xu M, McGehee DS, 2009. Stress-induced changes in nucleus accumbens glutamate synaptic plasticity. J Neurophysiol 101, 3192–3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannizzaro E, Cirrincione L, Mazzucco W, Scorciapino A, Catalano C, Ramaci T, Ledda C, Plescia F, 2020. Night-Time Shift Work and Related Stress Responses: A Study on Security Guards. Int J Environ Res Public Health 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon WB. Bodily Changes in Pain, Hunger, Fear and Rage. New York, NY: D. Appleton & Company; 1915. [Google Scholar]
- Cantet JM, Yu Z, Rius AG, 2021. Heat Stress-Mediated Activation of Immune-Inflammatory Pathways. Antibiotics (Basel) 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey RM, Padia, Shetal H, 2018. Chapter 1 - Physiology and Regulation of the Renin– Angiotensin–Aldosterone System, in: Singh AK, William, Gordon H. (Ed.), Textbook of Nephro-Endocrinology. Academic Press, pp. 1–25. [Google Scholar]
- Carrasco GA, Van de Kar LD, 2003. Neuroendocrine pharmacology of stress. Eur J Pharmacol 463, 235–272. [DOI] [PubMed] [Google Scholar]
- Carroll RG, 2007. 13 - Endocrine System. Mosby. [Google Scholar]
- Carter CS, Kenkel WM, MacLean EL, Wilson SR, Perkeybile AM, Yee JR, Ferris CF, Nazarloo HP, Porges SW, Davis JM, Connelly JJ, Kingsbury MA, 2020. Is Oxytocin "Nature's Medicine"? Pharmacol Rev 72, 829–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Perkeybile AM, 2018. The Monogamy Paradox: What Do Love and Sex Have to Do With It? Front Ecol Evol 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter JR, Goldstein DS, 2015. Sympathoneural and adrenomedullary responses to mental stress. Compr Physiol 5, 119–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso AJ, Chodzko-Zajko WJ, Bidinger DA, Sommers RK, 1994. Adults who stutter: responses to cognitive stress. J Speech Hear Res 37, 746–754. [DOI] [PubMed] [Google Scholar]
- Charney DS, 2004. Psychobiological mechanisms of resilience and vulnerability: implications for successful adaptation to extreme stress. Am J Psychiatry 161, 195–216. [DOI] [PubMed] [Google Scholar]
- Charney DS, Heninger GR, Jatlow PI, 1985. Increased anxiogenic effects of caffeine in panic disorders. Arch Gen Psychiatry 42, 233–243. [DOI] [PubMed] [Google Scholar]
- Chavanne AV, Robinson OJ, 2021. The Overlapping Neurobiology of Induced and Pathological Anxiety: A Meta-Analysis of Functional Neural Activation. Am J Psychiatry 178, 156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemical C, 2023. Yohimbine (hydrochloride). Cayman Chemical. [Google Scholar]
- Cheshire WP Jr., 2016. Thermoregulatory disorders and illness related to heat and cold stress. Auton Neurosci 196, 91–104. [DOI] [PubMed] [Google Scholar]
- Chikanza IC, Petrou P, Chrousos G, 2000. Perturbations of arginine vasopressin secretion during inflammatory stress. Pathophysiologic implications. Ann N Y Acad Sci 917, 825–834. [DOI] [PubMed] [Google Scholar]
- Christ-Crain M, Ball S, 2000. The Neurohypophysis: Endocrinology of Vasopressin and Oxytocin, in: Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya K, Dungan K, Hofland J, Kalra S, Kaltsas G, Kapoor N, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrere B, Levy M, McGee EA, McLachlan R, New M, Purnell J, Sahay R, Shah AS, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP (Eds.), Endotext, South Dartmouth (MA). [PubMed] [Google Scholar]
- Chrousos GP, 2009. Stress and disorders of the stress system. Nat Rev Endocrinol 5, 374–381. [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Gold PW, 1992. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA 267, 1244–1252. [PubMed] [Google Scholar]
- Cimolai N, Cimolai T, 2011. Yohimbine use for physical enhancement and its potential toxicity. J Diet Suppl 8, 346–354. [DOI] [PubMed] [Google Scholar]
- Cochran DM, Fallon D, Hill M, Frazier JA, 2013. The role of oxytocin in psychiatric disorders: a review of biological and therapeutic research findings. Harv Rev Psychiatry 21, 219–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R, 1983. A global measure of perceived stress. J Health Soc Behav 24, 385–396. [PubMed] [Google Scholar]
- Cohen S, Kessler RC, Gordon LU, 1995. Strategies for measuring stress in studies of psychiatric and physical disorders., Oxford University Press. [Google Scholar]
- Cohen S, Tyrrell DA, Smith AP, 1993. Negative life events, perceived stress, negative affect, and susceptibility to the common cold. J Pers Soc Psychol 64, 131–140. [DOI] [PubMed] [Google Scholar]
- Cohen S, Williamson GM, 1991. Stress and infectious disease in humans. Psychol Bull 109, 5–24. [DOI] [PubMed] [Google Scholar]
- Colten HR, Altevogt BM, Institute of Medicine. Committee on Sleep, M., Research, 2006. Sleep disorders and sleep deprivation : an unmet public health problem. Institute of Medicine : National Academies Press, Washington, D.C. [PubMed] [Google Scholar]
- Compton RJ, Hofheimer J, Kazinka R, 2013. Stress regulation and cognitive control: evidence relating cortisol reactivity and neural responses to errors. Cogn Affect Behav Neurosci 13, 152–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coote JH, 2005. A role for the paraventricular nucleus of the hypothalamus in the autonomic control of heart and kidney. Exp Physiol 90, 169–173. [DOI] [PubMed] [Google Scholar]
- Cramer MN, Jay O, 2016. Biophysical aspects of human thermoregulation during heat stress. Auton Neurosci 196, 3–13. [DOI] [PubMed] [Google Scholar]
- Crook J, Rideout E, Browne G, 1984. The prevalence of pain complaints in a general population. Pain 18, 299–314. [DOI] [PubMed] [Google Scholar]
- Crosswell AD, Lockwood KG, 2020. Best practices for stress measurement: How to measure psychological stress in health research. Health Psychol Open 7, 2055102920933072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzzo B, Padala SA, Lappin SL, 2023. Physiology, Vasopressin, StatPearls, Treasure Island (FL). [PubMed] [Google Scholar]
- Czapla-Iskrzycka A, Swiatkowska-Stodulska R, Sworczak K, 2022. Comorbidities in Mild Autonomous Cortisol Secretion - A Clinical Review of Literature. Exp Clin Endocrinol Diabetes 130, 567–576. [DOI] [PubMed] [Google Scholar]
- Danilescu CM, Ionescu M, Sandulescu DL, Pirlog MC, Streba CT, Rogoveanu I, 2022. Perceived Stress in Hepatitis C Virus Infected Patients under the DAA-Based Therapy. Diagnostics (Basel) 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskalakis NP, Meijer OC, de Kloet ER, 2022. Mineralocorticoid receptor and glucocorticoid receptor work alone and together in cell-type-specific manner: Implications for resilience prediction and targeted therapy. Neurobiol Stress 18, 100455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies A, Akerman AP, Rehrer NJ, Thornton SN, Cotter JD, 2023. Limited Effect of Dehydrating via Active vs. Passive Heat Stress on Plasma Volume or Osmolality, Relative to the Effect of These Stressors per Se. Nutrients 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daviu N, Bruchas MR, Moghaddam B, Sandi C, Beyeler A, 2019. Neurobiological links between stress and anxiety. Neurobiol Stress 11, 100191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Filippi G, 1991. [Pediatric radiology: between the devil and the deep sea]. Radiol Med 82, 10–12. [PubMed] [Google Scholar]
- de Kloet CS, Vermetten E, Geuze E, Kavelaars A, Heijnen CJ, Westenberg HG, 2006. Assessment of HPA-axis function in posttraumatic stress disorder: pharmacological and non-pharmacological challenge tests, a review. J Psychiatr Res 40, 550–567. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, 2003. Hormones, brain and stress. Endocr Regul 37, 51–68. [PubMed] [Google Scholar]
- de Kloet ER, de Kloet SF, de Kloet CS, de Kloet AD, 2019. Top-down and bottom-up control of stress-coping. J Neuroendocrinol 31, e12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet ER, Joels M, Holsboer F, 2005. Stress and the brain: from adaptation to disease. Nat Rev Neurosci 6, 463–475. [DOI] [PubMed] [Google Scholar]
- Dedovic K, D'Aguiar C, Pruessner JC, 2009. What stress does to your brain: a review of neuroimaging studies. Can J Psychiatry 54, 6–15. [DOI] [PubMed] [Google Scholar]
- Dedovic K, Renwick R, Mahani NK, Engert V, Lupien SJ, Pruessner JC, 2005. The Montreal Imaging Stress Task: using functional imaging to investigate the effects of perceiving and processing psychosocial stress in the human brain. J Psychiatry Neurosci 30, 319–325. [PMC free article] [PubMed] [Google Scholar]
- DePierro J, Lepow L, Feder A, Yehuda R, 2019. Translating Molecular and Neuroendocrine Findings in Posttraumatic Stress Disorder and Resilience to Novel Therapies. Biol Psychiatry 86, 454–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhabhar FS, 2009. Enhancing versus suppressive effects of stress on immune function: implications for immunoprotection and immunopathology. Neuroimmunomodulation 16, 300–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson K, Lehmann C, 2019. Inflammatory Response to Different Toxins in Experimental Sepsis Models. Int J Mol Sci 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Difede J, McAleavey AA, Emrich M, Jick A, Ovalles A, Wyka K, Spielman L, Olden M, Peskin M, Becket-Davenport C, Rubenstein A, Brownstein MJ, Damiano E, Itzkowitz D, Lu SF, Needell NJ, Kocsis JH, Gordon-Elliott JS, Simon NG, 2023. A proof-of- concept randomized crossover clinical trial of a first-in-class vasopressin 1a receptor antagonist for PTSD: Design, methods, and recruitment. Contemp Clin Trials Commun 33, 101116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, Li J, Cui L, Wang Y, Lin J, Qu Y, Wang H, 2018. Cortisol modulates inflammatory responses in LPS-stimulated RAW264.7 cells via the NF-kappaB and MAPK pathways. BMC Vet Res 14, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DrugBank, 2023. Dobutamine. Drug Bank. [Google Scholar]
- Dudek KA, Dion-Albert L, Kaufmann FN, Tuck E, Lebel M, Menard C, 2021. Neurobiology of resilience in depression: immune and vascular insights from human and animal studies. Eur J Neurosci 53, 183–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn AJ, 1993a. Infection as a stressor: a cytokine-mediated activation of the hypothalamo-pituitary-adrenal axis? Ciba Found Symp 172, 226–239; discussion 239–242. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, 1993b. Role of cytokines in infection-induced stress. Ann N Y Acad Sci 697, 189–202. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Berridge CW, 1990. Physiological and behavioral responses to corticotropin-releasing factor administration: is CRF a mediator of anxiety or stress responses? Brain Res Brain Res Rev 15, 71–100. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Powell ML, Meitin C, Small PA Jr., 1989. Virus infection as a stressor: influenza virus elevates plasma concentrations of corticosterone, and brain concentrations of MHPG and tryptophan. Physiol Behav 45, 591–594. [DOI] [PubMed] [Google Scholar]
- Dunn J, Grider MH, 2023. Physiology, Adenosine Triphosphate, StatPearls, Treasure Island (FL). [PubMed] [Google Scholar]
- Dunser MW, Hasibeder WR, 2009. Sympathetic overstimulation during critical illness: adverse effects of adrenergic stress. J Intensive Care Med 24, 293–316. [DOI] [PubMed] [Google Scholar]
- Dziurkowska E, Wesolowski M, 2021. Cortisol as a Biomarker of Mental Disorder Severity. J Clin Med 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrhart-Bornstein M, Hinson JP, Bornstein SR, Scherbaum WA, Vinson GP, 1998. Intraadrenal interactions in the regulation of adrenocortical steroidogenesis. Endocr Rev 19, 101–143. [DOI] [PubMed] [Google Scholar]
- El-Farhan N, Rees DA, Evans C, 2017. Measuring cortisol in serum, urine and saliva - are our assays good enough? Ann Clin Biochem 54, 308–322. [DOI] [PubMed] [Google Scholar]
- Elhendy A, Bax JJ, Poldermans D, 2002. Dobutamine stress myocardial perfusion imaging in coronary artery disease. J Nucl Med 43, 1634–1646. [PubMed] [Google Scholar]
- Elman I, Adler CM, Malhotra AK, Bir C, Pickar D, Breier A, 1998. Effect of acute metabolic stress on pituitary-adrenal axis activation in patients with schizophrenia. Am J Psychiatry 155, 979–981. [DOI] [PubMed] [Google Scholar]
- Elman I, Ariely D, Mazar N, Aharon I, Lasko NB, Macklin ML, Orr SP, Lukas SE, Pitman RK, 2005. Probing reward function in post-traumatic stress disorder with beautiful facial images. Psychiatry Res 135, 179–183. [DOI] [PubMed] [Google Scholar]
- Elman I, Becerra L, Tschibelu E, Yamamoto R, George E, Borsook D, 2012. Yohimbine-induced amygdala activation in pathological gamblers: a pilot study. PLoS One 7, e31118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elman I, Borsook D, 2016. Common Brain Mechanisms of Chronic Pain and Addiction. Neuron 89, 11–36. [DOI] [PubMed] [Google Scholar]
- Elman I, Borsook D, 2018. Threat Response System: Parallel Brain Processes in Pain vis-a-vis Fear and Anxiety. Front Psychiatry 9, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elman I, Borsook D, 2019. The failing cascade: Comorbid post traumatic stress- and opioid use disorders. Neurosci Biobehav Rev 103, 374–383. [DOI] [PubMed] [Google Scholar]
- Elman I, Borsook D, 2022. Comorbid opioid use disorder and post-traumatic stress disorder. Lancet Psychiatry 9, e28–e29. [DOI] [PubMed] [Google Scholar]
- Elman I, Borsook D, Lukas SE, 2006. Food intake and reward mechanisms in patients with schizophrenia: implications for metabolic disturbances and treatment with second-generation antipsychotic agents. Neuropsychopharmacology 31, 2091–2120. [DOI] [PubMed] [Google Scholar]
- Elman I, Borsook D, Volkow ND, 2013. Pain and suicidality: insights from reward and addiction neuroscience. Prog Neurobiol 109, 1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elman I, Breier A, 1997. Effects of acute metabolic stress on plasma progesterone and testosterone in male subjects: relationship to pituitary-adrenocortical axis activation. Life Sci 61, 1705–1712. [DOI] [PubMed] [Google Scholar]
- Elman I, D'Ambra MN, Krause S, Breiter H, Kane M, Morris R, Tuffy L, Gastfriend DR, 2001a. Ultrarapid opioid detoxification: effects on cardiopulmonary physiology, stress hormones and clinical outcomes. Drug Alcohol Depend 61, 163–172. [DOI] [PubMed] [Google Scholar]
- Elman I, Goldstein DS, Adler CM, Shoaf SE, Breier A, 2001b. Inverse relationship between plasma epinephrine and testosterone levels during acute glucoprivation in healthy men. Life Sci 68, 1889–1898. [DOI] [PubMed] [Google Scholar]
- Elman I, Howard M, Borodovsky JT, Mysels D, Rott D, Borsook D, Albanese M, 2020. Metabolic and Addiction Indices in Patients on Opioid Agonist Medication-Assisted Treatment: A Comparison of Buprenorphine and Methadone. Sci Rep 10, 5617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elman I, Krause S, Breiter HC, Gollub RL, Heintges J, Baumgartner WA, Rosen BR, Gastfriend DR, 2000. The validity of self-reported drug use in non-treatment seeking individuals with cocaine dependence: correlation with biochemical assays. Am J Addict 9, 216–221. [DOI] [PubMed] [Google Scholar]
- Elman I, Lowen S, Frederick BB, Chi W, Becerra L, Pitman RK, 2009. Functional neuroimaging of reward circuitry responsivity to monetary gains and losses in posttraumatic stress disorder. Biol Psychiatry 66, 1083–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elman I, Lukas S, Shoaf SE, Rott D, Adler C, Breier A, 2003. Effects of acute metabolic stress on the peripheral vasopressinergic system in schizophrenia. J Psychopharmacol 17, 317–323. [DOI] [PubMed] [Google Scholar]
- Elman I, Rott D, Green AI, Langleben DD, Lukas SE, Goldstein DS, Breier A, 2004. Effects of pharmacological doses of 2-deoxyglucose on plasma catecholamines and glucose levels in patients with schizophrenia. Psychopharmacology (Berl) 176, 369–375. [DOI] [PubMed] [Google Scholar]
- Elman I, Tschibelu E, Borsook D, 2010. Psychosocial stress and its relationship to gambling urges in individuals with pathological gambling. Am J Addict 19, 332–339. [DOI] [PubMed] [Google Scholar]
- Elman I, Upadhyay J, Langleben DD, Albanese M, Becerra L, Borsook D, 2018. Reward and aversion processing in patients with post-traumatic stress disorder: functional neuroimaging with visual and thermal stimuli. Transl Psychiatry 8, 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elman I, Zubieta JK, Borsook D, 2011. The missing p in psychiatric training: why it is important to teach pain to psychiatrists. Arch Gen Psychiatry 68, 12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmert MH, Herman JP, 1999. Differential forebrain c-fos mRNA induction by ether inhalation and novelty: evidence for distinctive stress pathways. Brain Res 845, 60–67. [DOI] [PubMed] [Google Scholar]
- Encyclopedia NW, 2023. Epinephrine, New World Encyclopedia. [Google Scholar]
- Epstein DH, Preston KL, Stewart J, Shaham Y, 2006. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl) 189, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdozain AM, Penagarikano O, 2019. Oxytocin as Treatment for Social Cognition, Not There Yet. Front Psychiatry 10, 930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etgen AM, 1995. Oxytocin does not act locally in the hypothalamus to elicit norepinephrine release. Brain Res 703, 242–244. [DOI] [PubMed] [Google Scholar]
- Feder A, Nestler EJ, Charney DS, 2009. Psychobiology and molecular genetics of resilience. Nat Rev Neurosci 10, 446–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson AV, Latchford KJ, Samson WK, 2008. The paraventricular nucleus of the hypothalamus - a potential target for integrative treatment of autonomic dysfunction. Expert Opin Ther Targets 12, 717–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira GS, Veening-Griffioen DH, Boon WPC, Moors EHM, Gispen-de Wied CC, Schellekens H, van Meer PJK, 2019. A standardised framework to identify optimal animal models for efficacy assessment in drug development. PLoS One 14, e0218014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer S, 2021. The hypothalamus in anxiety disorders. Handb Clin Neurol 180, 149–160. [DOI] [PubMed] [Google Scholar]
- Fischer S, Schumacher T, Knaevelsrud C, Ehlert U, Schumacher S, 2021. Genes and hormones of the hypothalamic-pituitary-adrenal axis in post-traumatic stress disorder. What is their role in symptom expression and treatment response? J Neural Transm (Vienna) 128, 1279–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flesch M, Hoper A, Dell'Italia L, Evans K, Bond R, Peshock R, Diwan A, Brinsa TA, Wei CC, Sivasubramanian N, Spinale FG, Mann DL, 2003. Activation and functional significance of the renin-angiotensin system in mice with cardiac restricted overexpression of tumor necrosis factor. Circulation 108, 598–604. [DOI] [PubMed] [Google Scholar]
- Floras JS, 2009. Sympathetic nervous system activation in human heart failure: clinical implications of an updated model. J Am Coll Cardiol 54, 375–385. [DOI] [PubMed] [Google Scholar]
- Flory JD, Yehuda R, 2015. Comorbidity between post-traumatic stress disorder and major depressive disorder: alternative explanations and treatment considerations. Dialogues Clin Neurosci 17, 141–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follenius M, Brandenberger G, Oyono S, Candas V, 1982. Cortisol as a sensitive index of heat-intolerance. Physiol Behav 29, 509–513. [DOI] [PubMed] [Google Scholar]
- Forstenpointner J, Elman I, Freeman R, Borsook D, 2022. The omnipresence of autonomic modulation in health and disease. Prog Neurobiol 210, 102218. [DOI] [PubMed] [Google Scholar]
- Fountain JH, Kaur J, Lappin SL, 2023. Physiology, Renin Angiotensin System, StatPearls, Treasure Island (FL). [PubMed] [Google Scholar]
- Franco AJ, Chen C, Scullen T, Zsombok A, Salahudeen AA, Di S, Herman JP, Tasker JG, 2016. Sensitization of the Hypothalamic-Pituitary-Adrenal Axis in a Male Rat Chronic Stress Model. Endocrinology 157, 2346–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricke K, Alexander N, Jacobsen T, Krug H, Wehkamp K, Vogel S, 2023. The effects of hydrocortisone and yohimbine on human behavior in approach-avoidance conflicts. Psychopharmacology (Berl) 240, 1705–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch JU, Hausser JA, Mojzisch A, 2015. The Trier Social Stress Test as a paradigm to study how people respond to threat in social interactions. Front Psychol 6, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisone F, Sicari F, Settineri S, Merlo EM, Clinical Psychological Assessment of Stress: A Narrative Review of the Last 5 Years. Clin Neuropsychiatry. 2021. Apr;18(2):91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friuli M, Eramo B, Valenza M, Scuderi C, Provensi G, Romano A, 2021. Targeting the Oxytocinergic System: A Possible Pharmacological Strategy for the Treatment of Inflammation Occurring in Different Chronic Diseases. Int J Mol Sci 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Q, 2022. Autonomic dysfunction and cardiovascular risk in post-traumatic stress disorder. Auton Neurosci 237, 102923. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Flugge G, 2004. Cellular consequences of stress and depression. Dialogues Clin Neurosci 6, 171–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuhara K, Kvetnansky R, Cizza G, Pacak K, Ohara H, Goldstein DS, Kopin IJ, 1996. Interrelations between sympathoadrenal system and hypothalamo-pituitary-adrenocortical/thyroid systems in rats exposed to cold stress. J Neuroendocrinol 8, 533–541. [DOI] [PubMed] [Google Scholar]
- Gaab J, Rohleder N, Nater UM, Ehlert U, 2005. Psychological determinants of the cortisol stress response: the role of anticipatory cognitive appraisal. Psychoneuroendocrinology 30, 599–610. [DOI] [PubMed] [Google Scholar]
- Gabrys RL, Howell JW, Cebulski SF, Anisman H, Matheson K, 2019. Acute stressor effects on cognitive flexibility: mediating role of stressor appraisals and cortisol. Stress 22, 182–189. [DOI] [PubMed] [Google Scholar]
- Galea S, 2017. Chronic Pain and the Health of Populations, Dean’s Notes. Boston University School of Public Health, Boston. [Google Scholar]
- Ganesan S, Reynolds C, Hollinger K, Pearce SC, Gabler NK, Baumgard LH, Rhoads RP, Selsby JT, 2016. Twelve hours of heat stress induces inflammatory signaling in porcine skeletal muscle. Am J Physiol Regul Integr Comp Physiol 310, R1288–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gann DS, 1979. Cortisol secretion after hemorrhage: multiple mechanisms. Nephron 23, 119–124. [DOI] [PubMed] [Google Scholar]
- Ganong WF, 1981. NEUROENDOCRINE RESPONSES TO INJURY AND SHOCK. Pergamon. [Google Scholar]
- Ganong WF, Murakami K, 1987. The role of angiotensin II in the regulation of ACTH secretion. Ann N Y Acad Sci 512, 176–186. [DOI] [PubMed] [Google Scholar]
- Gardani M, Bradford DRR, Russell K, Allan S, Beattie L, Ellis JG, Akram U, 2022. A systematic review and meta-analysis of poor sleep, insomnia symptoms and stress in undergraduate students. Sleep Med Rev 61, 101565. [DOI] [PubMed] [Google Scholar]
- Gareau MG, Wine E, Rodrigues DM, Cho JH, Whary MT, Philpott DJ, Macqueen G, Sherman PM, 2011. Bacterial infection causes stress-induced memory dysfunction in mice. Gut 60, 307–317. [DOI] [PubMed] [Google Scholar]
- Garzon-Villalba XP, Wu Y, Ashley CD, Bernard TE, 2017. Ability to Discriminate Between Sustainable and Unsustainable Heat Stress Exposures-Part 2: Physiological Indicators. Ann Work Expo Health 61, 621–632. [DOI] [PubMed] [Google Scholar]
- Gauer R, Meyers BK, 2019. Heat-Related Illnesses. Am Fam Physician 99, 482–489. [PubMed] [Google Scholar]
- Ghaddar A, Omar KH, Dokmak M, Kansour NA, Jbara Z, Laham S, Ali S, 2014. Work-related stress and urinary catecholamines among laboratory technicians. J Occup Health 55, 398–404. [DOI] [PubMed] [Google Scholar]
- Gibbons CH, Adler GK, Bonyhay I, Freeman R, 2012. Experimental hypoglycemia is a human model of stress-induced hyperalgesia. Pain 153, 2204–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gideon A, Sauter C, Fieres J, Berger T, Renner B, Wirtz PH, 2020. Kinetics and Interrelations of the Renin Aldosterone Response to Acute Psychosocial Stress: A Neglected Stress System. J Clin Endocrinol Metab 105, e762–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert P, Allan S, 1998. The role of defeat and entrapment (arrested flight) in depression: an exploration of an evolutionary view. Psychol Med 28, 585–598. [DOI] [PubMed] [Google Scholar]
- Godin PJ, Fleisher LA, Eidsath A, Vandivier RW, Preas HL, Banks SM, Buchman TG, Suffredini AF, 1996. Experimental human endotoxemia increases cardiac regularity: results from a prospective, randomized, crossover trial. Crit Care Med 24, 1117–1124. [DOI] [PubMed] [Google Scholar]
- Godinho-Silva C, Cardoso F, Veiga-Fernandes H, 2019. Neuro-Immune Cell Units: A New Paradigm in Physiology. Annu Rev Immunol 37, 19–46. [DOI] [PubMed] [Google Scholar]
- Godoy LD, Rossignoli MT, Delfino-Pereira P, Garcia-Cairasco N, de Lima Umeoka EH, 2018. A Comprehensive Overview on Stress Neurobiology: Basic Concepts and Clinical Implications. Front Behav Neurosci 12, 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel MU, Mills PJ, Irwin MR, Ziegler MG, 2000. Interleukin-6 and tumor necrosis factor-alpha production after acute psychological stress, exercise, and infused isoproterenol: differential effects and pathways. Psychosom Med 62, 591–598. [DOI] [PubMed] [Google Scholar]
- Gola H, Engler H, Schauer M, Adenauer H, Riether C, Kolassa S, Elbert T, Kolassa IT, 2012. Victims of rape show increased cortisol responses to trauma reminders: a study in individuals with war- and torture-related PTSD. Psychoneuroendocrinology 37, 213–220. [DOI] [PubMed] [Google Scholar]
- Gold PW, Chrousos GP, 2002. Organization of the stress system and its dysregulation in melancholic and atypical depression: high vs low CRH/NE states. Mol Psychiatry 7, 254–275. [DOI] [PubMed] [Google Scholar]
- Goldstein DS, 1981. Plasma norepinephrine in essential hypertension. A study of the studies. Hypertension 3, 48–52. [DOI] [PubMed] [Google Scholar]
- Goldstein DS, 1995. Stress, catecholamines and cardiovascular disease. Oxford University Press, New York. [Google Scholar]
- Goldstein DS, 2010. Adrenal responses to stress. Cell Mol Neurobiol 30, 1433–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DS, 2021. Stress and the "extended" autonomic system. Auton Neurosci 236, 102889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DS, Kopin IJ, 2007. Evolution of concepts of stress. Stress 10, 109–120. [DOI] [PubMed] [Google Scholar]
- Goldstein DS, Kopin IJ, 2008. Adrenomedullary, adrenocortical, and sympathoneural responses to stressors: a meta-analysis. Endocr Regul 42, 111–119. [PMC free article] [PubMed] [Google Scholar]
- Gomez-Sanchez E, Gomez-Sanchez CE, 2014. The multifaceted mineralocorticoid receptor. Compr Physiol 4, 965–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez JA, Beller GA, 2017. Choosing exercise or pharmacologic stress imaging, or exercise ECG testing alone: How to decide. J Nucl Cardiol 24, 555–557. [DOI] [PubMed] [Google Scholar]
- Gothert M, 1985. Role of autoreceptors in the function of the peripheral and central nervous system. Arzneimittelforschung 35, 1909–1916. [PubMed] [Google Scholar]
- Gottlieb SS, Dilsizian V, Wawrzyniak AJ, Smith M, Harris KM, Whittaker K, Krantz D, 2014. Congruity between Mental Stress-Induced and Adenosine-Induced Myocardial Ischemia Assessed Using SPECT in Heart Failure Patients. CARDIOVASCULAR PHYSIOLOGY I 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green CL, Nahhas RW, Scoglio AA, Elman I, 2017. Post-traumatic stress symptoms in pathological gambling: Potential evidence of anti-reward processes. J Behav Addict 6, 98–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoric P, 2007. The Sensory Apparatus, in Aristotle on the common sense. Oxford University Press [Google Scholar]
- Gresch PJ, Sved AF, Zigmond MJ, Finlay JM, 1994. Stress-induced sensitization of dopamine and norepinephrine efflux in medial prefrontal cortex of the rat. J Neurochem 63, 575–583. [DOI] [PubMed] [Google Scholar]
- Grewen KM, Girdler SS, Amico J, Light KC, 2005. Effects of partner support on resting oxytocin, cortisol, norepinephrine, and blood pressure before and after warm partner contact. Psychosom Med 67, 531–538. [DOI] [PubMed] [Google Scholar]
- Griep EN, Boersma JW, Lentjes EG, Prins AP, van der Korst JK, de Kloet ER, 1998. Function of the hypothalamic-pituitary-adrenal axis in patients with fibromyalgia and low back pain. J Rheumatol 25, 1374–1381. [PubMed] [Google Scholar]
- Grigoleit JS, Kullmann JS, Wolf OT, Hammes F, Wegner A, Jablonowski S, Engler H, Gizewski E, Oberbeck R, Schedlowski M, 2011. Dose-dependent effects of endotoxin on neurobehavioral functions in humans. PLoS One 6, e28330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinage BD, 2003. Diagnosis and management of post-traumatic stress disorder. Am Fam Physician 68, 2401–2408. [PubMed] [Google Scholar]
- Gross L, 2006. Avoiding punishment is its own reward. PLoS Biol 4, e247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Group C-NC, 1992. The changing rate of major depression. Cross-national comparisons. JAMA 268, 3098–3105. [DOI] [PubMed] [Google Scholar]
- Grunhaus L, Tiongco D, Zelnik T, Flegel P, Hollingsworth PJ, Smith CB, 1989. Intravenous yohimbine. Selective enhancer of norepinephrine and cortisol secretion and systolic blood pressure in humans. Clin Neuropharmacol 12, 106–114. [DOI] [PubMed] [Google Scholar]
- Guieu R, Deharo JC, Maille B, Crotti L, Torresani E, Brignole M, Parati G, 2020. Adenosine and the Cardiovascular System: The Good and the Bad. J Clin Med 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunes Ozunal Z, Sabirli S, Sen S, Karamursel S, & Omer B, 2021. Renin-angiotensin system in stress response and the effect of chronic exercise in healthy volunteers. Annals of Medical Research 27, 988–992. [Google Scholar]
- Gurantz D, Cowling RT, Villarreal FJ, Greenberg BH, 1999. Tumor necrosis factor-alpha upregulates angiotensin II type 1 receptors on cardiac fibroblasts. Circ Res 85, 272–279. [DOI] [PubMed] [Google Scholar]
- Hannibal KE, Bishop MD, 2014. Chronic stress, cortisol dysfunction, and pain: a psychoneuroendocrine rationale for stress management in pain rehabilitation. Phys Ther 94, 1816–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JA, Huecker MR, 2023. Sleep Deprivation, StatPearls, Treasure Island (FL). [Google Scholar]
- Haruki Y, Ogawa K, 2021. Role of anatomical insular subdivisions in interoception: Interoceptive attention and accuracy have dissociable substrates. Eur J Neurosci 53, 2669–2680. [DOI] [PubMed] [Google Scholar]
- Havenith G, Griggs K, Qiu Y, Dorman L, Kulasekaran V, Hodder S, 2020. Higher comfort temperature preferences for anthropometrically matched Chinese and Japanese versus white-western-middle-European individuals using a personal comfort / cooling system. Building and Environment 183. [Google Scholar]
- Hellhammer J, Schubert M, 2012. The physiological response to Trier Social Stress Test relates to subjective measures of stress during but not before or after the test. Psychoneuroendocrinology 37, 119–124. [DOI] [PubMed] [Google Scholar]
- Henn FA, Vollmayr B, 2005. Stress models of depression: forming genetically vulnerable strains. Neurosci Biobehav Rev 29, 799–804. [DOI] [PubMed] [Google Scholar]
- Herd SA, Banich MT, O'Reilly RC, 2006. Neural mechanisms of cognitive control: an integrative model of stroop task performance and FMRI data. J Cogn Neurosci 18, 22–32. [DOI] [PubMed] [Google Scholar]
- Herman JP, 2012. Neural pathways of stress integration: relevance to alcohol abuse. Alcohol Res 34, 441–447. [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE, 1997. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci 20, 78–84. [DOI] [PubMed] [Google Scholar]
- Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE, 2003. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol 24, 151–180. [DOI] [PubMed] [Google Scholar]
- Herman JP, McKlveen JM, Ghosal S, Kopp B, Wulsin A, Makinson R, Scheimann J, Myers B, 2016. Regulation of the Hypothalamic-Pituitary-Adrenocortical Stress Response. Compr Physiol 6, 603–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinotsu K, Miyaji C, Yada Y, Kawai H, Sakamoto S, Okahisa Y, Tsutsui K, Kanbayashi T, Tanaka K, Takao S, Kishi Y, Takaki M, Yamada N, 2022. The validity of atypical psychosis diagnostic criteria to detect anti-NMDA receptor encephalitis with psychiatric symptoms. Schizophr Res 248, 292–299. [DOI] [PubMed] [Google Scholar]
- Hirotsu C, Tufik S, Andersen ML, 2015. Interactions between sleep, stress, and metabolism: From physiological to pathological conditions. Sleep Sci 8, 143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho JM, Blevins JE, 2013. Coming full circle: contributions of central and peripheral oxytocin actions to energy balance. Endocrinology 154, 589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holleman GA, Hooge ITC, Kemner C, Hessels RS, 2020. The 'Real-World Approach' and Its Problems: A Critique of the Term Ecological Validity. Front Psychol 11, 721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmberg G, Gershon S, 1961. Autonomic and psychic effects of yohimbine hydrochloride. Psychopharmacologia 2, 93–106. [DOI] [PubMed] [Google Scholar]
- Hopper JW, Pitman RK, Su Z, Heyman GM, Lasko NB, Macklin ML, Orr SP, Lukas SE, Elman I, 2008. Probing reward function in posttraumatic stress disorder: expectancy and satisfaction with monetary gains and losses. J Psychiatr Res 42, 802–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton RW, Meldrum BS, Bachelard HS, 1973. Enzymic and cerebral metabolic effects of 2-deoxy-D-glucose. J Neurochem 21, 507–520. [DOI] [PubMed] [Google Scholar]
- Howe AS, Boden BP, 2007. Heat-related illness in athletes. Am J Sports Med 35, 1384–1395. [DOI] [PubMed] [Google Scholar]
- Hu HY, Mokuda O, Sakamoto Y, Shimizu N, 1992. Direct effect of ACTH on renin release in isolated perfused guinea-pig kidneys with adrenal glands. Acta Endocrinol (Copenh) 127, 142–145. [DOI] [PubMed] [Google Scholar]
- Huang WL, Liao SC, Gau SS, 2021. Association between Stroop tasks and heart rate variability features in patients with somatic symptom disorder. J Psychiatr Res 136, 246–255. [DOI] [PubMed] [Google Scholar]
- IASP, I.A.f.t.S.o.P., 2020. IASP Announces Revised Definition of Pain. International Association for the Study of Pain. [Google Scholar]
- Ibarra-Coronado EG, Pantaleon-Martinez AM, Velazquez-Moctezuma J, Prospero-Garcia O, Mendez-Diaz M, Perez-Tapia M, Pavon L, Morales-Montor J, 2015. The Bidirectional Relationship between Sleep and Immunity against Infections. J Immunol Res 2015, 678164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iftikhar K, Siddiq A, Baig SG, Zehra S, 2020. Substance P: A neuropeptide involved in the psychopathology of anxiety disorders. Neuropeptides 79, 101993. [DOI] [PubMed] [Google Scholar]
- Insel TR, 2003. Is social attachment an addictive disorder? Physiol Behav 79, 351–357. [DOI] [PubMed] [Google Scholar]
- Iob E, Baldwin JR, Plomin R, Steptoe A, 2021. Adverse childhood experiences, daytime salivary cortisol, and depressive symptoms in early adulthood: a longitudinal genetically informed twin study. Transl Psychiatry 11, 420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iovino M, Messana T, Marucci S, Triggiani D, Giagulli VA, Guastamacchia E, Piazzolla G, De Pergola G, Lisco G, Triggiani V, 2023. The neurohypophyseal hormone oxytocin and eating behaviors: a narrative review. Hormones (Athens). [DOI] [PMC free article] [PubMed]
- Ishikawa Y, Kitaoka S, Kawano Y, Ishii S, Suzuki T, Wakahashi K, Kato T, Katayama Y, Furuyashiki T, 2021. Repeated social defeat stress induces neutrophil mobilization in mice: maintenance after cessation of stress and strain-dependent difference in response. Br J Pharmacol 178, 827–844. [DOI] [PubMed] [Google Scholar]
- Ivell R, Balvers M, Rust W, Bathgate R, Einspanier A, 1997. Oxytocin and male reproductive function. Adv Exp Med Biol 424, 253–264. [DOI] [PubMed] [Google Scholar]
- Jameson J, F.A., Kasper DL, Hauser SL, Longo DL, Loscalzo J, 2018. Harrison's principles of internal medicine, 20th Edition. McGraw Hill, New York. [Google Scholar]
- Janak PH, Tye KM, 2015. From circuits to behaviour in the amygdala. Nature 517, 284–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janbek J, Laursen TM, Frimodt-Moller N, Magyari M, Haas JG, Lathe R, Waldemar G, 2023. Hospital-Diagnosed Infections, Autoimmune Diseases, and Subsequent Dementia Incidence. JAMA Netw Open 6, e2332635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen SA, Arntz A, Bouts S, 1998. Anxiety and pain: epinephrine-induced hyperalgesia and attentional influences. Pain 76, 309–316. [DOI] [PubMed] [Google Scholar]
- Jasper MS, Engeland WC, 1997. Splanchnicotomy increases adrenal sensitivity to ACTH in nonstressed rats. Am J Physiol 273, E363–368. [DOI] [PubMed] [Google Scholar]
- Jedema HP, Sved AF, Zigmond MJ, Finlay JM, 1999. Sensitization of norepinephrine release in medial prefrontal cortex: effect of different chronic stress protocols. Brain Res 830, 211–217. [DOI] [PubMed] [Google Scholar]
- Jensen AR, Rohwer WD Jr., 1966. The Stroop color-word test: a review. Acta Psychol (Amst) 25, 36–93. [DOI] [PubMed] [Google Scholar]
- Jezova D, Skultetyova I, Tokarev DI, Bakos P, Vigas M, 1995. Vasopressin and oxytocin in stress. Ann N Y Acad Sci 771, 192–203. [DOI] [PubMed] [Google Scholar]
- Ji Y, Shi W, Yang J, Ma B, Jin T, Cao B, Liu X, Ma K, 2022. Effect of sympathetic sprouting on the excitability of dorsal root ganglion neurons and afferents in a rat model of neuropathic pain. Biochem Biophys Res Commun 587, 49–57. [DOI] [PubMed] [Google Scholar]
- Jirikowski GF, Ochs SD, Caldwell JD, 2018. Oxytocin and Steroid Actions. Curr Top Behav Neurosci 35, 77–95. [DOI] [PubMed] [Google Scholar]
- Jochberger S, Dorler J, Luckner G, Mayr VD, Wenzel V, Ulmer H, Morgenthaler NG, Hasibeder WR, Dunser MW, 2009. The vasopressin and copeptin response to infection, severe sepsis, and septic shock. Crit Care Med 37, 476–482. [DOI] [PubMed] [Google Scholar]
- Joels M, Sarabdjitsingh RA, den Boon FS, Karst H,, 2017. Chapter 33 - Rapid and Slow Effects of Corticosteroid Hormones on Hippocampal Activity, in: Fink G. (Ed.), Stress: Neuroendocrinology and Neurobiology, Academic Press, pp. 327–341. [Google Scholar]
- Jones KR, Myers B, Herman JP, 2011. Stimulation of the prelimbic cortex differentially modulates neuroendocrine responses to psychogenic and systemic stressors. Physiol Behav 104, 266–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph NT, Jiang Y, Zilioli S, 2021. Momentary emotions and salivary cortisol: A systematic review and meta-analysis of ecological momentary assessment studies. Neurosci Biobehav Rev 125, 365–379. [DOI] [PubMed] [Google Scholar]
- Joyner MJ, Casey DP, 2015. Regulation of increased blood flow (hyperemia) to muscles during exercise: a hierarchy of competing physiological needs. Physiol Rev 95, 549–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juruena MF, 2014. Early-life stress and HPA axis trigger recurrent adulthood depression. Epilepsy Behav 38, 148–159. [DOI] [PubMed] [Google Scholar]
- Justiz Vaillant AA, Qurie A, 2023. Interleukin, StatPearls, Treasure Island (FL). [Google Scholar]
- Kahn H, 2002. On Paradigms, Theories and Models. Problemas del Desarrollo 34. [Google Scholar]
- Kalisch R, Baker DG, Basten U, Boks MP, Bonanno GA, Brummelman E, Chmitorz A, Fernandez G, Fiebach CJ, Galatzer-Levy I, Geuze E, Groppa S, Helmreich I, Hendler T, Hermans EJ, Jovanovic T, Kubiak T, Lieb K, Lutz B, Muller MB, Murray RJ, Nievergelt CM, Reif A, Roelofs K, Rutten BPF, Sander D, Schick A, Tuscher O, Diest IV, Harmelen AV, Veer IM, Vermetten E, Vinkers CH, Wager TD, Walter H, Wessa M, Wibral M, Kleim B, 2017. The resilience framework as a strategy to combat stress-related disorders. Nat Hum Behav 1, 784–790. [DOI] [PubMed] [Google Scholar]
- Kamperis K, Hagstroem S, Radvanska E, Rittig S, Djurhuus JC, 2010. Excess diuresis and natriuresis during acute sleep deprivation in healthy adults. Am J Physiol Renal Physiol 299, F404–411. [DOI] [PubMed] [Google Scholar]
- Kanaide H, Ichiki T, Nishimura J, Hirano K, 2003. Cellular mechanism of vasoconstriction induced by angiotensin II: it remains to be determined. Circ Res 93, 1015–1017. [DOI] [PubMed] [Google Scholar]
- Karatsoreos IN, McEwen BS, 2013. Resilience and vulnerability: a neurobiological perspective. F1000Prime Rep 5, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsgodt KH, Lukas SE, Elman I, 2003. Psychosocial stress and the duration of cocaine use in non-treatment seeking individuals with cocaine dependence. Am J Drug Alcohol Abuse 29, 539–551. [DOI] [PubMed] [Google Scholar]
- Kato M, Phillips BG, Sigurdsson G, Narkiewicz K, Pesek CA, Somers VK, 2000. Effects of sleep deprivation on neural circulatory control. Hypertension 35, 1173–1175. [DOI] [PubMed] [Google Scholar]
- Katrinli S, Oliveira NCS, Felger JC, Michopoulos V, Smith AK, 2022. The role of the immune system in posttraumatic stress disorder. Transl Psychiatry 12, 313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kausche FM, Zerbes G, Kampermann L, Muller JC, Wiedemann K, Buchel C, Schwabe L, 2021. Noradrenergic stimulation increases fear memory expression. Eur Neuropsychopharmacol 43, 71–81. [DOI] [PubMed] [Google Scholar]
- Keim S, Curtis AB, Belardinelli L, Epstein ML, Staples ED, Lerman BB, 1992. Adenosine-induced atrioventricular block: a rapid and reliable method to assess surgical and radiofrequency catheter ablation of accessory atrioventricular pathways. J Am Coll Cardiol 19, 1005–1012. [DOI] [PubMed] [Google Scholar]
- Kerr D, MacDonald IA, Tattersall RB, 1989. Influence of duration of hypoglycemia on the hormonal counterregulatory response in normal subjects. J Clin Endocrinol Metab 68, 1118–1122. [DOI] [PubMed] [Google Scholar]
- Khoury NM, Marvar PJ, Gillespie CF, Wingo A, Schwartz A, Bradley B, Kramer M, Ressler KJ, 2012. The renin-angiotensin pathway in posttraumatic stress disorder: angiotensin-converting enzyme inhibitors and angiotensin receptor blockers are associated with fewer traumatic stress symptoms. J Clin Psychiatry 73, 849–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim A, Knudsen JG, Madara JC, Benrick A, Hill TG, Abdul Kadir L, Kellard JA, Mellander L, Miranda C, Lin H, James T, Suba K, Spigelman AF, Wu Y, MacDonald PE, Wernstedt Asterholm I, Magnussen T, Christensen M, Vilsboll T, Salem V, Knop FK, Rorsman P, Lowell BB, Briant LJ, 2021. Arginine-vasopressin mediates counterregulatory glucagon release and is diminished in type 1 diabetes. Elife 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Lee J, Boone D, 2022. Protective and Risk Factors at the Intersection of Chronic Pain, Depression, Anxiety, and Somatic Amplification: A Latent Profile Approach. J Pain Res 15, 1107–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King VL, English VL, Bharadwaj K, Cassis LA, 2013. Angiotensin II Stimulates Sympathetic Neurotransmission to Adipose Tissue. Physiol Rep 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinlein SA, Wilson CD, Karatsoreos IN, 2015. Dysregulated hypothalamic-pituitary-adrenal axis function contributes to altered endocrine and neurobehavioral responses to acute stress. Front Psychiatry 6, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney KL, Rao U, Bailey B, Hellman N, Kelly C, McAfee NW, Morris MC, 2023. Dynamics of diurnal cortisol and alpha-amylase secretion and their associations with PTSD onset in recent interpersonal trauma survivors. Psychol Med 53, 2263–2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH, 1993. The 'Trier Social Stress Tesť--a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology 28, 76–81. [DOI] [PubMed] [Google Scholar]
- Kirschbaum CD, Oliver; Gehrke Jörg; Wüst Stefan, 1991. Cortisol and Behavior: The “Trier Mental Challenge Test” (TMCT) — First Evaluation of a New Psychological Stress Test.
- Koldzic-Zivanovic N, Tu H, Juelich TL, Rady PL, Tyring SK, Hudnall SD, Smith EM, Hughes TK, 2006. Regulation of adrenal glucocorticoid synthesis by interleukin-10: a preponderance of IL-10 receptor in the adrenal zona fasciculata. Brain Behav Immun 20, 460–468. [DOI] [PubMed] [Google Scholar]
- Koob GF, Schulkin J, 2019. Addiction and stress: An allostatic view. Neurosci Biobehav Rev 106, 245–262. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, Coppens CM, de Boer SF, Buwalda B, Meerlo P, Timmermans PJ, 2013. The resident-intruder paradigm: a standardized test for aggression, violence and social stress. J Vis Exp, e4367. [DOI] [PMC free article] [PubMed]
- Kopp UC, DiBona GF, 1986. Interaction between epinephrine and renal nerves in control of renin secretion rate. Am J Physiol 250, F999–1007. [DOI] [PubMed] [Google Scholar]
- Kosunen KJ, Pakarinen AJ, Kuoppasalmi K, Adlercreutz H, 1976. Plasma renin activity, angiotensin II, and aldosterone during intense heat stress. J Appl Physiol 41, 323–327. [DOI] [PubMed] [Google Scholar]
- Kramer LB, Whiteman SE, Petri JM, Spitzer EG, Weathers FW, 2023. Self-Rated Versus Clinician-Rated Assessment of Posttraumatic Stress Disorder: An Evaluation of Discrepancies Between the PTSD Checklist for DSM-5 and the Clinician-Administered PTSD Scale for DSM-5. Assessment 30, 1590–1605. [DOI] [PubMed] [Google Scholar]
- Kumar A, Rinwa P, Kaur G, Machawal L, 2013. Stress: Neurobiology, consequences and management. J Pharm Bioallied Sci 5, 91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz-Plapp T, 2018. Subjective heat stress in everyday life. ESKP Earth System Knowledge Platform. [Google Scholar]
- Kunzel H, Schussler P, Yassouridis A, Uhr M, Kluge M, Steiger A, 2020. The renin secretion profile under the influence of sleep deprivation and the neuropeptides CRH and GHRH. Psychoneuroendocrinology 120, 104799. [DOI] [PubMed] [Google Scholar]
- Kvetnansky R, Pacak K, Fukuhara K, Viskupic E, Hiremagalur B, Nankova B, Goldstein DS, Sabban EL, Kopin IJ, 1995. Sympathoadrenal system in stress. Interaction with the hypothalamic-pituitary-adrenocortical system. Ann N Y Acad Sci 771, 131–158. [DOI] [PubMed] [Google Scholar]
- Laceulle OM, Nederhof E, van Aken MAG, Ormel J, 2017. Adversity-driven changes in hypothalamic-pituitary-adrenal axis functioning during adolescence. The trails study. Psychoneuroendocrinology 85, 49–55. [DOI] [PubMed] [Google Scholar]
- Lago TR, Brownstein MJ, Page E, Beydler E, Manbeck A, Beale A, Roberts C, Balderston N, Damiano E, Pineles SL, Simon N, Ernst M, Grillon C, 2021. The novel vasopressin receptor (V1aR) antagonist SRX246 reduces anxiety in an experimental model in humans: a randomized proof-of-concept study. Psychopharmacology (Berl) 238, 2393–2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam JA, Murray ER, Yu KE, Ramsey M, Nguyen TT, Mishra J, Martis B, Thomas ML, Lee EE, 2021. Neurobiology of loneliness: a systematic review. Neuropsychopharmacology 46, 1873–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamotte G, Shouman K, Benarroch EE, 2021. Stress and central autonomic network. Auton Neurosci 235, 102870. [DOI] [PubMed] [Google Scholar]
- Landis E, Shore E, 1989. Yohimbine-induced bronchospasm. Chest 96, 1424. [DOI] [PubMed] [Google Scholar]
- Laskawiec D, Grajek M, Szlacheta P, Korzonek-Szlacheta I, 2022. Post-Pandemic Stress Disorder as an Effect of the Epidemiological Situation Related to the COVID-19 Pandemic. Healthcare (Basel) 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviola G, Adriani W, Rea M, Aloe L, Alleva E, 2004. Social withdrawal, neophobia, and stereotyped behavior in developing rats exposed to neonatal asphyxia. Psychopharmacology (Berl) 175, 196–205. [DOI] [PubMed] [Google Scholar]
- Le JT, Watson P, Begg D, Albertella L, Le Pelley ME, 2021. Physiological and subjective validation of a novel stress procedure: The Simple Singing Stress Procedure. Behav Res Methods 53, 1478–1487. [DOI] [PubMed] [Google Scholar]
- Lee S, Craddock Z, Rivier C, 2011. Brain stem catecholamines circuitry: activation by alcohol and role in the hypothalamic-pituitary-adrenal response to this drug. J Neuroendocrinol 23, 531–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefton J, 2023. The Safety Concerns and Uses of Yohimbe: Yohimbe risks may outweigh possible benefits, in: Nieves M. (Ed.), Herbal Supplements. VeryWell Health. [Google Scholar]
- Leibowitz SF, Eidelman D, Suh JS, Diaz S, Sladek CD, 1990. Mapping study of noradrenergic stimulation of vasopressin release. Exp Neurol 110, 298–305. [DOI] [PubMed] [Google Scholar]
- Lerner JS, Li Y, Valdesolo P, Kassam KS, 2015. Emotion and decision making. Annu Rev Psychol 66, 799–823. [DOI] [PubMed] [Google Scholar]
- Levy ME, Waters A, Sen S, Castel AD, Plankey M, Molock S, Asch F, Goparaju L, Kassaye S, 2021. Psychosocial stress and neuroendocrine biomarker concentrations among women living with or without HIV. PLoS One 16, e0261746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough TC, Choe H, Farzan M, 2003. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 426, 450–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilly MP, Engeland WC, Gann DS, 1983. Responses of cortisol secretion to repeated hemorrhage in the anesthetized dog. Endocrinology 112, 681–688. [DOI] [PubMed] [Google Scholar]
- Lincoln SH, Johnson T, Laquidara JR, Wilt J, Obeid R, 2022. Increased rates of social defeat and schizotypy in racial minorities. Personality and Individual Differences Volume 186. [Google Scholar]
- Linden CH, Vellman WP, Rumack B, 1985. Yohimbine: a new street drug. Ann Emerg Med 14, 1002–1004. [DOI] [PubMed] [Google Scholar]
- Lionakis MS, Kontoyiannis DP, 2003. Glucocorticoids and invasive fungal infections. Lancet 362, 1828–1838. [DOI] [PubMed] [Google Scholar]
- Lippert TH, Mueck AO, Seeger H, Pfaff A, 2003. Effects of oxytocin outside pregnancy. Horm Res 60, 262–271. [DOI] [PubMed] [Google Scholar]
- Litvin Y, Murakami G, Pfaff DW, 2011. Effects of chronic social defeat on behavioral and neural correlates of sociality: Vasopressin, oxytocin and the vasopressinergic V1b receptor. Physiol Behav 103, 393–403. [DOI] [PubMed] [Google Scholar]
- Liu C, Chu D, Kalantar-Zadeh K, George J, Young HA, Liu G, 2021. Cytokines: From Clinical Significance to Quantification. Adv Sci (Weinh) 8, e2004433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YZ, Wang YX, Jiang CL, 2017. Inflammation: The Common Pathway of Stress-Related Diseases. Front Hum Neurosci 11, 316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louie P, Wu C, Shahidi FV, Siddiqi A. Inflation hardship, gender, and mental health. SSM Popul Health. 2023. Jun 12;23:101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love TM, 2018. The impact of oxytocin on stress: the role of sex. Curr Opin Behav Sci 23, 136–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovibond PF, Lovibond SH, 1995. The structure of negative emotional states: comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behav Res Ther 33, 335–343. [DOI] [PubMed] [Google Scholar]
- Lowder SC, Hamet P, Liddle GW, 1976. Contrasting effects of hypoglycemia on plasma renin activity and cyclic adenosine 3',5'-monophosphate (cyclic AMP) in low renin and normal renin essential hypertension. Circ Res 38, 105–108. [DOI] [PubMed] [Google Scholar]
- Lowe SR, Galea S, 2017. The Mental Health Consequences of Mass Shootings. Trauma Violence Abuse 18, 62–82. [DOI] [PubMed] [Google Scholar]
- Lowe TW, 1990. Hypovolemia due to hemorrhage. Clin Obstet Gynecol 33, 454–459. [DOI] [PubMed] [Google Scholar]
- Lundberg U, 2007. Catecholamines, in: Fink G. (Ed.), Encyclopedia of Stress (Second Edition). Academic Press, pp. 419–423. [Google Scholar]
- Lusardi P, Zoppi A, Preti P, Pesce RM, Piazza E, Fogari R, 1999. Effects of insufficient sleep on blood pressure in hypertensive patients: a 24-h study. Am J Hypertens 12, 63–68. [DOI] [PubMed] [Google Scholar]
- Lyte M, Li W, Opitz N, Gaykema RP, Goehler LE, 2006. Induction of anxiety-like behavior in mice during the initial stages of infection with the agent of murine colonic hyperplasia Citrobacter rodentium. Physiol Behav 89, 350–357. [DOI] [PubMed] [Google Scholar]
- Ma K, Gao W, Xu H, Liang W, Ma G, 2022. Role and Mechanism of the Renin-Angiotensin-Aldosterone System in the Onset and Development of Cardiorenal Syndrome. J Renin Angiotensin Aldosterone Syst 2022, 3239057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma TK, Kam KK, Yan BP, Lam YY, 2010. Renin-angiotensin-aldosterone system blockade for cardiovascular diseases: current status. Br J Pharmacol 160, 1273–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M, Minner B, Suy E, Vandervorst C, Raus J, 1991. Coexisting dysregulations of both the sympathoadrenal system and hypothalamic-pituitary-adrenal-axis in melancholia. J Neural Transm Gen Sect 85, 195–210. [DOI] [PubMed] [Google Scholar]
- Mahler B, Kamperis K, Schroeder M, Frokiaer J, Djurhuus JC, Rittig S, 2012. Sleep deprivation induces excess diuresis and natriuresis in healthy children. Am J Physiol Renal Physiol 302, F236–243. [DOI] [PubMed] [Google Scholar]
- Maragos WF, Jakel RJ, Pang Z, Geddes JW, 1998. 6-Hydroxydopamine injections into the nigrostriatal pathway attenuate striatal malonate and 3-nitropropionic acid lesions. Exp Neurol 154, 637–644. [DOI] [PubMed] [Google Scholar]
- Marini F, Pozzato C, Andreetta V, Jansson B, Arban R, Domenici E, Carboni L, 2006. Single exposure to social defeat increases corticotropin-releasing factor and glucocorticoid receptor mRNA expression in rat hippocampus. Brain Res 1067, 25–35. [DOI] [PubMed] [Google Scholar]
- Martin BJ, Chen HI, 1984. Sleep loss and the sympathoadrenal response to exercise. Med Sci Sports Exerc 16, 56–59. [PubMed] [Google Scholar]
- Marzvanyan A, Alhawaj AF, 2023. Physiology, Sensory Receptors, StatPearls, Treasure Island (FL). [PubMed] [Google Scholar]
- Masood K, Ahmed B, Choi J, Gutierrez-Osuna R, 2012. Consistency and validity of self-reporting scores in stress measurement surveys. Annu Int Conf IEEE Eng Med Biol Soc 2012, 4895–4898. [DOI] [PubMed] [Google Scholar]
- Matsuzaki K, Upton D, 2013. Wound treatment and pain management: a stressful time. Int Wound J 10, 638–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavani GP, DeVita MV, Michelis MF, 2015. A review of the nonpressor and nonantidiuretic actions of the hormone vasopressin. Front Med (Lausanne) 2, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazloumi A, Golbabaei F, Mahmood Khani S, Kazemi Z, Hosseini M, Abbasinia M, Farhang Dehghan S, 2014. Evaluating Effects of Heat Stress on Cognitive Function among Workers in a Hot Industry. Health Promot Perspect 4, 240–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann SM, Antunes-Rodrigues J, Franci CR, Anselmo-Franci JA, Karanth S, Rettori V, 2000. Role of the hypothalamic pituitary adrenal axis in the control of the response to stress and infection. Braz J Med Biol Res 33, 1121–1131. [DOI] [PubMed] [Google Scholar]
- McCorry LK, 2007. Physiology of the autonomic nervous system. Am J Pharm Educ 71, 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, 1998. Stress, adaptation, and disease. Allostasis and allostatic load. Ann N Y Acad Sci 840, 33–44. [DOI] [PubMed] [Google Scholar]
- McEwen BS, 2000. The neurobiology of stress: from serendipity to clinical relevance. Brain Res 886, 172–189. [DOI] [PubMed] [Google Scholar]
- McEwen BS, 2007. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev 87, 873–904. [DOI] [PubMed] [Google Scholar]
- McEwen BS, 2020. Hormones and behavior and the integration of brain-body science. Horm Behav 119, 104619. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Akil H, 2020. Revisiting the Stress Concept: Implications for Affective Disorders. J Neurosci 40, 12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ, 2010. Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Ann N Y Acad Sci 1186, 190–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ, 2011. Stress- and allostasis-induced brain plasticity. Annu Rev Med 62, 431–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Kalia M, 2010. The role of corticosteroids and stress in chronic pain conditions. Metabolism 59 Suppl 1, S9–15. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Karatsoreos IN, 2015. Sleep Deprivation and Circadian Disruption: Stress, Allostasis, and Allostatic Load. Sleep Med Clin 10, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Wingfield JC, 2003. The concept of allostasis in biology and biomedicine. Horm Behav 43, 2–15. [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Hatzenbuehler ML, 2009. Stressful life events, anxiety sensitivity, and internalizing symptoms in adolescents. J Abnorm Psychol 118, 659–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMorris T, Swain J, Smith M, Corbett J, Delves S, Sale C, Harris RC, Potter J, 2006. Heat stress, plasma concentrations of adrenaline, noradrenaline, 5-hydroxytryptamine and cortisol, mood state and cognitive performance. Int J Psychophysiol 61, 204–215. [DOI] [PubMed] [Google Scholar]
- MedlinePlus, 2021. Hypovolemic Shock, in: Boork J. (Ed.). National Library of Medicine, Bethesda, MD. [Google Scholar]
- Melendez Rivera JG, Anjum F, 2023. Hypovolemia, StatPearls, Treasure Island (FL). [PubMed] [Google Scholar]
- Menezes FP, Da Silva RS, 2017. Chapter 22 - Caffeine. Academic Press. [Google Scholar]
- Mercer ME, Holder MD, 1997. Antinociceptive effects of palatable sweet ingesta on human responsivity to pressure pain. Physiol Behav 61, 311–318. [DOI] [PubMed] [Google Scholar]
- Michie HR, Manogue KR, Spriggs DR, Revhaug A, O'Dwyer S, Dinarello CA, Cerami A, Wolff SM, Wilmore DW, 1988. Detection of circulating tumor necrosis factor after endotoxin administration. N Engl J Med 318, 1481–1486. [DOI] [PubMed] [Google Scholar]
- Milham MP, Banich MT, Claus ED, Cohen NJ, 2003. Practice-related effects demonstrate complementary roles of anterior cingulate and prefrontal cortices in attentional control. Neuroimage 18, 483–493. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Sorge RE, LaCroix-Fralish ML, Smith SB, Fortin A, Sotocinal SG, Ritchie J, Austin JS, Schorscher-Petcu A, Melmed K, Czerminski J, Bittong RA, Mokris JB, Neubert JK, Campbell CM, Edwards RR, Campbell JN, Crawley JN, Lariviere WR, Wallace MR, Sternberg WF, Balaban CD, Belfer I, Fillingim RB, 2011. Pain sensitivity and vasopressin analgesia are mediated by a gene-sex-environment interaction. Nat Neurosci 14, 1569–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohiyeddini C, Bauer S, Semple S, 2013. Displacement behaviour is associated with reduced stress levels among men but not women. PLoS One 8, e56355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokuda O, Sakamoto Y, Kawagoe R, Ubukata E, Shimizu N, 1992. Epinephrine augments cortisol secretion from isolated perfused adrenal glands of guinea pigs. Am J Physiol 262, E806–809. [DOI] [PubMed] [Google Scholar]
- Moller K, Strauss GI, Qvist J, Fonsmark L, Knudsen GM, Larsen FS, Krabbe KS, Skinhoj P, Pedersen BK, 2002. Cerebral blood flow and oxidative metabolism during human endotoxemia. J Cereb Blood Flow Metab 22, 1262–1270. [DOI] [PubMed] [Google Scholar]
- Montain SJ, Laird JE, Latzka WA, Sawka MN, 1997. Aldosterone and vasopressin responses in the heat: hydration level and exercise intensity effects. Med Sci Sports Exerc 29, 661–668. [DOI] [PubMed] [Google Scholar]
- Morais-Silva G, Costa-Ferreira W, Gomes-de-Souza L, Pavan JC, Crestani CC, Marin MT, 2019. Cardiovascular outcomes related to social defeat stress: New insights from resilient and susceptible rats. Neurobiol Stress 11, 100181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales A, 2000. Yohimbine in erectile dysfunction: the facts. Int J Impot Res 12 Suppl 1, S70–74. [PubMed] [Google Scholar]
- Morris A, Patel G, 2023. Heat Stroke, StatPearls, Treasure Island (FL). [Google Scholar]
- Morris LS, McCall JG, Charney DS, Murrough JW, 2020. The role of the locus coeruleus in the generation of pathological anxiety. Brain Neurosci Adv 4, 2398212820930321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottola F, Gnisci A, Kalaitzaki A, Vintila M, Sergi I, 2023. The impact of the Russian-Ukrainian war on the mental health of Italian people after 2 years of the pandemic: risk and protective factors as moderators. Front Psychol 14, 1154502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueser KT, Salyers MP, Rosenberg SD, Ford JD, Fox L, Carty P, 2001. Psychometric evaluation of trauma and posttraumatic stress disorder assessments in persons with severe mental illness. Psychol Assess 13, 110–117. [DOI] [PubMed] [Google Scholar]
- Muhtz C, Rodriguez-Raecke R, Hinkelmann K, Moeller-Bertram T, Kiefer F, Wiedemann K, May A, Otte C, 2013. Cortisol response to experimental pain in patients with chronic low back pain and patients with major depression. Pain Med 14, 498–503. [DOI] [PubMed] [Google Scholar]
- Muneer M, 2021. Hypoglycaemia. Adv Exp Med Biol 1307, 43–69. [DOI] [PubMed] [Google Scholar]
- Munshi S, Ritger A, Rosenkranz AJ, 2022. Induction of Repeated Social Defeat Stress in Rats. Bio Protoc 12, e4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murck H, Held K, Ziegenbein M, Kunzel H, Koch K, Steiger A, 2003. The renin-angiotensin-aldosterone system in patients with depression compared to controls--a sleep endocrine study. BMC Psychiatry 3, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murck H, Schussler P, Steiger A, 2012. Renin-angiotensin-aldosterone system: the forgotten stress hormone system: relationship to depression and sleep. Pharmacopsychiatry 45, 83–95. [DOI] [PubMed] [Google Scholar]
- Nagai M, Iriki M, 2001. Changes in Immune Activities by Heat Stress. Springer, Tokyo. [Google Scholar]
- Nakatake Y, Furuie H, Yamada M, Kuniishi H, Ukezono M, Yoshizawa K, Yamada M, 2020. The effects of emotional stress are not identical to those of physical stress in mouse model of social defeat stress. Neurosci Res 158, 56–63. [DOI] [PubMed] [Google Scholar]
- Narvaez Linares NF, Charron V, Ouimet AJ, Labelle PR, Plamondon H, 2020. A systematic review of the Trier Social Stress Test methodology: Issues in promoting study comparison and replicable research. Neurobiol Stress 13, 100235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann ID, 2002. Involvement of the brain oxytocin system in stress coping: interactions with the hypothalamo-pituitary-adrenal axis. Prog Brain Res 139, 147–162. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Kromer SA, Toschi N, Ebner K, 2000. Brain oxytocin inhibits the (re)activity of the hypothalamo-pituitary-adrenal axis in male rats: involvement of hypothalamic and limbic brain regions. Regul Pept 96, 31–38. [DOI] [PubMed] [Google Scholar]
- Nicolaides NC, Pavlaki AN, Maria Alexandra MA, Chrousos GP, 2000. Glucocorticoid Therapy and Adrenal Suppression, in: Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya K, Dungan K, Hofland J, Kalra S, Kaltsas G, Kapoor N, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrere B, Levy M, McGee EA, McLachlan R, New M, Purnell J, Sahay R, Shah AS, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP (Eds.), Endotext, South Dartmouth (MA). [PubMed] [Google Scholar]
- Niraula A, Wang Y, Godbout JP, Sheridan JF, 2018. Corticosterone Production during Repeated Social Defeat Causes Monocyte Mobilization from the Bone Marrow, Glucocorticoid Resistance, and Neurovascular Adhesion Molecule Expression. J Neurosci 38, 2328–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura H, Yoshimura M, Shimizu M, Sanada K, Sonoda S, Nishimura K, Baba K, Ikeda N, Motojima Y, Maruyama T, Nonaka Y, Baba R, Onaka T, Horishita T, Morimoto H, Yoshida Y, Kawasaki M, Sakai A, Muratani M, Conway-Campbell B, Lightman S, Ueta Y, 2022. Endogenous oxytocin exerts anti-nociceptive and anti-inflammatory effects in rats. Commun Biol 5, 907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nollet M, Wisden W, Franks NP, 2020. Sleep deprivation and stress: a reciprocal relationship. Interface Focus 10, 20190092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor DB, Thayer JF, Vedhara K, 2021. Stress and Health: A Review of Psychobiological Processes. Annu Rev Psychol 72, 663–688. [DOI] [PubMed] [Google Scholar]
- O'Keefe JH Jr., Bateman TM, Silvestri R, Barnhart C, 1992. Safety and diagnostic accuracy of adenosine thallium-201 scintigraphy in patients unable to exercise and those with left bundle branch block. Am Heart J 124, 614–621. [DOI] [PubMed] [Google Scholar]
- OĽeary R, Penrose H, Miyata K, Satou R, 2016. Macrophage-derived IL-6 contributes to ANG II-mediated angiotensinogen stimulation in renal proximal tubular cells. Am J Physiol Renal Physiol 310, F1000–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olpe HR, Steinmann M, 1991. Responses of locus coeruleus neurons to neuropeptides. Prog Brain Res 88, 241–248. [DOI] [PubMed] [Google Scholar]
- Osorio FL, Loureiro SR, Hallak JEC, Machado-de-Sousa JP, Ushirohira JM, Baes CVW, Apolinario TD, Donadon MF, Bolsoni LM, Guimaraes T, Fracon VS, Silva-Rodrigues APC, Pizeta FA, Souza RM, Sanches RF, Dos Santos RG, Martin-Santos R, Crippa JAS, 2019. Clinical validity and intrarater and test-retest reliability of the Structured Clinical Interview for DSM-5 - Clinician Version (SCID-5-CV). Psychiatry Clin Neurosci 73, 754–760. [DOI] [PubMed] [Google Scholar]
- Pacurari M, Kafoury R, Tchounwou PB, Ndebele K, 2014. The Renin-Angiotensin-aldosterone system in vascular inflammation and remodeling. Int J Inflam 2014, 689360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer BW, Shir C, Chang H, Mulvaney M, Hall JMH, Shu IW, Jin H, Lohr JB, 2021. Increased Prevalence of Metabolic Syndrome in Veterans with PTSD Untreated with Antipsychotic Medications. Int J Ment Health 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X, Kaminga AC, Wen SW, Liu A, 2018. Catecholamines in Post-traumatic Stress Disorder: A Systematic Review and Meta-Analysis. Front Mol Neurosci 11, 450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadimitriou A, Priftis KN, 2009. Regulation of the hypothalamic-pituitary-adrenal axis. Neuroimmunomodulation 16, 265–271. [DOI] [PubMed] [Google Scholar]
- Park J, Eo EY., Lee KH, Park JS, Lee JH, Yoo CG, Lee CT, Cho YJ, 2015. The Anti-Inflammatory Effect of Arginine-Vasopressin on Lipopolysaccharide-Induced IκBα/Nuclear Factor-κB Cascade. Korean Journal of Critical Care Medicine 30, 151–157. [Google Scholar]
- Parris BA, Hasshim N, Wadsley M, Augustinova M, Ferrand L, 2022. The loci of Stroop effects: a critical review of methods and evidence for levels of processing contributing to color-word Stroop effects and the implications for the loci of attentional selection. Psychol Res 86, 1029–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SR, Zhu X, Storfer-Isser A, Mehra R, Jenny NS, Tracy R, Redline S, 2009. Sleep duration and biomarkers of inflammation. Sleep 32, 200–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pego JM, Sousa JC, Almeida OF, Sousa N, 2010. Stress and the neuroendocrinology of anxiety disorders. Curr Top Behav Neurosci 2, 97–117. [DOI] [PubMed] [Google Scholar]
- Perianez JA, Lubrini G, Garcia-Gutierrez A, Rios-Lago M, 2021. Construct Validity of the Stroop Color-Word Test: Influence of Speed of Visual Search, Verbal Fluency, Working Memory, Cognitive Flexibility, and Conflict Monitoring. Arch Clin Neuropsychol 36, 99–111. [DOI] [PubMed] [Google Scholar]
- Persson PB, 2003. Renin: origin, secretion and synthesis. J Physiol 552, 667–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pervanidou P, Kolaitis G, Charitaki S, Lazaropoulou C, Papassotiriou I, Hindmarsh P, Bakoula C, Tsiantis J, Chrousos GP, 2007. The natural history of neuroendocrine changes in pediatric posttraumatic stress disorder (PTSD) after motor vehicle accidents: progressive divergence of noradrenaline and cortisol concentrations over time. Biol Psychiatry 62, 1095–1102. [DOI] [PubMed] [Google Scholar]
- Peskind ER, Veith RC, Dorsa DM, Gumbrecht G, Raskind MA, 1989. Yohimbine increases cerebrospinal fluid and plasma norepinephrine but not arginine vasopressin in humans. Neuroendocrinology 50, 286–291. [DOI] [PubMed] [Google Scholar]
- Pessoa L, 2010. Emotion and cognition and the amygdala: from "what is it?" to "whaťs to be done?". Neuropsychologia 48, 3416–3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie EC, Peskind ER, Dobie DJ, Veith RC, Raskind MA, 2000. Increased plasma norepinephrine response to yohimbine in elderly men. J Gerontol A Biol Sci Med Sci 55, M155–159. [DOI] [PubMed] [Google Scholar]
- Pfister SL, Keeton TK, 1988. The mechanism of yohimbine-induced renin release in the conscious rat. Naunyn Schmiedebergs Arch Pharmacol 337, 35–46. [DOI] [PubMed] [Google Scholar]
- Piazza JR, Almeida DM, Dmitrieva NO, Klein LC, 2010. Frontiers in the use of biomarkers of health in research on stress and aging. J Gerontol B Psychol Sci Soc Sci 65, 513–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Le Moal M, 1998. The role of stress in drug self-administration. Trends Pharmacol Sci 19, 67–74. [DOI] [PubMed] [Google Scholar]
- Pickworth KK, 1992. Long-term dobutamine therapy for refractory congestive heart failure. Clin Pharm 11, 618–624. [PubMed] [Google Scholar]
- Piedmont RL, 2014. Criterion Validity., in: Michalos AC (Ed.), Encyclopedia of Quality of Life and Well-Being Research. Springer, Dordrecht. [Google Scholar]
- Polad JE, Wilson LM, 2002. Myocardial infarction during adenosine stress test. Heart 87, E2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell DJ, Liossi C, Moss-Morris R, Schlotz W, 2013. Unstimulated cortisol secretory activity in everyday life and its relationship with fatigue and chronic fatigue syndrome: a systematic review and subset meta-analysis. Psychoneuroendocrinology 38, 2405–2422. [DOI] [PubMed] [Google Scholar]
- Prevor MB, Diamond A, 2005. Color-object interference in young children: A Stroop effect in children 3(1/2)-6(1/2) years old. Cogn Dev 20, 256–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price LH, Charney DS, Heninger GR, 1984. Three cases of manic symptoms following yohimbine administration. Am J Psychiatry 141, 1267–1268. [DOI] [PubMed] [Google Scholar]
- Price LH, Charney DS, Rubin AL, Heninger GR, 1986. Alpha 2-adrenergic receptor function in depression. The cortisol response to yohimbine. Arch Gen Psychiatry 43, 849–858. [DOI] [PubMed] [Google Scholar]
- Quaedflieg CW, Meyer T, Smeets T, 2013. The imaging Maastricht Acute Stress Test (iMAST): a neuroimaging compatible psychophysiological stressor. Psychophysiology 50, 758–766. [DOI] [PubMed] [Google Scholar]
- Raben A, Holst JJ, Christensen NJ, Astrup A, 1996. Determinants of postprandial appetite sensations: macronutrient intake and glucose metabolism. Int J Obes Relat Metab Disord 20, 161–169. [PubMed] [Google Scholar]
- Rajaei S, 2022. Cell-Mediated Immunity, in: Rezaei N. (Ed.), Encyclopedia of Infection and Immunity. Elsevier, pp. 56–63. [Google Scholar]
- Rajalingam D, Nymoen I, Nyberg H, Nielsen MB, Einarsen SV, Gjerstad J, 2021. Workplace bullying increases the risk of anxiety through a stress-induced beta2-adrenergic receptor mechanism: a multisource study employing an animal model, cell culture experiments and human data. Int Arch Occup Environ Health 94, 1905–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramphal-Naley L, 2012. Screening for heat stress in workers and athletes. Proc (Bayl Univ Med Cent) 25, 224–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao R, Androulakis IP, 2019. Allostatic adaptation and personalized physiological trade-offs in the circadian regulation of the HPA axis: A mathematical modeling approach. Sci Rep 9, 11212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razavi Nematollahi L, Kitabchi AE, Stentz FB, Wan JY, Larijani BA, Tehrani MM, Gozashti MH, Omidfar K, Taheri E, 2009. Proinflammatory cytokines in response to insulin-induced hypoglycemic stress in healthy subjects. Metabolism 58, 443–448. [DOI] [PubMed] [Google Scholar]
- Reaven GM, Lithell H, Landsberg L, 1996. Hypertension and associated metabolic abnormalities--the role of insulin resistance and the sympathoadrenal system. N Engl J Med 334, 374–381. [DOI] [PubMed] [Google Scholar]
- Rees SJ, Moussa B, 2023. Invisible wounds of the Israel-Gaza war in Australia. Med J Aust. [DOI] [PMC free article] [PubMed]
- Reichel CM, 2021. Unraveling oxytocin's peripheral vs. central mechanisms. Neuropsychopharmacology 46, 273–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichmann F, Holzer P, 2016. Neuropeptide Y: A stressful review. Neuropeptides 55, 99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rejeski WJ, Gagne M, Parker PE, Koritnik DR, 1989. Acute stress reactivity from contested dominance in dominant and submissive males. Behav Med 15, 118–124. [DOI] [PubMed] [Google Scholar]
- Renaud P, Blondin JP, 1997. The stress of Stroop performance: physiological and emotional responses to color-word interference, task pacing, and pacing speed. Int J Psychophysiol 27, 87–97. [DOI] [PubMed] [Google Scholar]
- Riad M, Mogos M, Thangathurai D, Lumb PD, 2002. Steroids. Curr Opin Crit Care 8, 281–284. [DOI] [PubMed] [Google Scholar]
- Richardt G, Waas W, Kranzhofer R, Cheng B, Lohse MJ, Schomig A, 1989. Interaction between the release of adenosine and noradrenaline during sympathetic stimulation: a feed-back mechanism in rat heart. J Mol Cell Cardiol 21, 269–277. [DOI] [PubMed] [Google Scholar]
- Rodrigues SM, LeDoux JE, Sapolsky RM, 2009. The influence of stress hormones on fear circuitry. Annu Rev Neurosci 32, 289–313. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Diaz R, Molano RD, Weitz JR, Abdulreda MH, Berman DM, Leibiger B, Leibiger IB, Kenyon NS, Ricordi C, Pileggi A, Caicedo A, Berggren PO, 2018. Paracrine Interactions within the Pancreatic Islet Determine the Glycemic Set Point. Cell Metab 27, 549–558 e544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde P, 2001. The relevance of hierarchies, territories, defeat for depression in humans: hypotheses and clinical predictions. J Affect Disord 65, 221–230. [DOI] [PubMed] [Google Scholar]
- Romanski LM, 2012. Convergence of Auditory, Visual, and Somatosensory Information in Ventral Prefrontal Cortex, in: Murray MM, Wallace MT (Eds.), The Neural Bases of Multisensory Processes, Boca Raton (FL). [PubMed] [Google Scholar]
- Rotenberg S, McGrath JJ, 2016. Inter-relation between autonomic and HPA axis activity in children and adolescents. Biol Psychol 117, 16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell JA, Fjell C, Hsu JL, Lee T, Boyd J, Thair S, Singer J, Patterson AJ, Walley KR, 2013. Vasopressin compared with norepinephrine augments the decline of plasma cytokine levels in septic shock. Am J Respir Crit Care Med 188, 356–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saab PG, Llabre MM, Hurwitz BE, Frame CA, Reineke LJ, Fins AI, McCalla J, Cieply LK, Schneiderman N, 1992. Myocardial and peripheral vascular responses to behavioral challenges and their stability in black and white Americans. Psychophysiology 29, 384–397. [DOI] [PubMed] [Google Scholar]
- Saab PG, Llabre MM, Hurwitz BE, Schneiderman N, Wohlgemuth W, Durel LA, Massie C, Nagel J, 1993. The cold pressor test: vascular and myocardial response patterns and their stability. Psychophysiology 30, 366–373. [DOI] [PubMed] [Google Scholar]
- Sahu MK, Dubey RK, Chandrakar A, Kumar M, Kumar M, 2022. A systematic review and meta-analysis of serum and plasma cortisol levels in depressed patients versus control. Indian J Psychiatry 64, 440–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salim Al-Hadramy M, Ali F, 1989. Catecholamines in Heat Stroke. Military Medicine 154, 263–264. [PubMed] [Google Scholar]
- Sallee FR, Sethuraman G, Sine L, Liu H, 2000. Yohimbine challenge in children with anxiety disorders. Am J Psychiatry 157, 1236–1242. [DOI] [PubMed] [Google Scholar]
- SAMHSA, S.A.a.M.H.S.A., 2023. Types of Disasters.
- Samuels ER, Szabadi E, 2008a. Functional neuroanatomy of the noradrenergic locus coeruleus: its roles in the regulation of arousal and autonomic function part I: principles of functional organisation. Curr Neuropharmacol 6, 235–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels ER, Szabadi E, 2008b. Functional neuroanatomy of the noradrenergic locus coeruleus: its roles in the regulation of arousal and autonomic function part II: physiological and pharmacological manipulations and pathological alterations of locus coeruleus activity in humans. Curr Neuropharmacol 6, 254–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels JD, Lotstein ML, Lehmann ML, Elkahloun AG, Banerjee S, Herkenham M, 2023. Chronic social defeat alters brain vascular-associated cell gene expression patterns leading to vascular dysfunction and immune system activation. J Neuroinflammation 20, 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU, 2000. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev 21, 55–89. [DOI] [PubMed] [Google Scholar]
- Satou R, Penrose H, Navar LG, 2018. Inflammation as a Regulator of the Renin-Angiotensin System and Blood Pressure. Curr Hypertens Rep 20, 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawka MN, 1992. Physiological consequences of hypohydration: exercise performance and thermoregulation. Med Sci Sports Exerc 24, 657–670. [PubMed] [Google Scholar]
- Sawka MN, Cheuvront SN, Kenefick RW, 2015. Hypohydration and Human Performance: Impact of Environment and Physiological Mechanisms. Sports Med 45 Suppl 1, S51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schadt JC, Ludbrook J, 1991. Hemodynamic and neurohumoral responses to acute hypovolemia in conscious mammals. Am J Physiol 260, H305–318. [DOI] [PubMed] [Google Scholar]
- Scheurink A, Ritter S, 1993. Sympathoadrenal responses to glucoprivation and lipoprivation in rats. Physiol Behav 53, 995–1000. [DOI] [PubMed] [Google Scholar]
- Schindler-Gmelch L, Capito K, Steudte-Schmiedgen S, Kirschbaum C, Berking M, 2023. Hair Cortisol Research in Posttraumatic Stress Disorder - 10 Years of Insights and Open Questions. A Systematic Review. Curr Neuropharmacol. [DOI] [PMC free article] [PubMed]
- Schlarb AA, Classen M, Hellmann SM, Vogele C, Gulewitsch MD, 2017. Sleep and somatic complaints in university students. J Pain Res 10, 1189–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlotz W, 2013. Stress Reactivity, in: Gellman MD, Turner J.Rick (Ed.), Encyclopedia of behavioral medicine. Springer, New York, NY, pp. 1891–1894. [Google Scholar]
- Schmidt MH, 2014. The energy allocation function of sleep: a unifying theory of sleep, torpor, and continuous wakefulness. Neurosci Biobehav Rev 47, 122–153. [DOI] [PubMed] [Google Scholar]
- Schuhmann BB, Henderson SN, Black RA, Van Hasselt VB, Klimley Margres K, Masias EV, LeDuc TJ, 2022. A Behavioral-Analytic Model for Assessing Stress in Firefighters. Behav Modif 46, 267–293. [DOI] [PubMed] [Google Scholar]
- Schwartz NS, Clutter WE, Shah SD, Cryer PE, 1987. Glycemic thresholds for activation of glucose counterregulatory systems are higher than the threshold for symptoms. J Clin Invest 79, 777–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scofield MD, Heinsbroek JA, Gipson CD, Kupchik YM, Spencer S, Smith AC, Roberts-Wolfe D, Kalivas PW, 2016. The Nucleus Accumbens: Mechanisms of Addiction across Drug Classes Reflect the Importance of Glutamate Homeostasis. Pharmacol Rev 68, 816–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See RE, Waters RP, 2010. Pharmacologically-induced stress: a cross-species probe for translational research in drug addiction and relapse. Am J Transl Res 3, 81–89. [PMC free article] [PubMed] [Google Scholar]
- Segato FN, Castro-Souza C, Segato EN, Morato S, Coimbra NC, 1997. Sucrose ingestion causes opioid analgesia. Braz J Med Biol Res 30, 981–984. [DOI] [PubMed] [Google Scholar]
- Segerstrom SC, Miller GE, 2004. Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychol Bull 130, 601–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekaninova N, Bona Olexova L, Visnovcova Z, Ondrejka I, Tonhajzerova I, 2020. Role of Neuroendocrine, Immune, and Autonomic Nervous System in Anorexia Nervosa-Linked Cardiovascular Diseases. Int J Mol Sci 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki K, Yoshida S, Jaiswal MK, 2018. Molecular mechanism of noradrenaline during the stress-induced major depressive disorder. Neural Regen Res 13, 1159–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seligowski AV, Duffy LA, Merker JB, Michopoulos V, Gillespie CF, Marvar PJ, Stein MB, Ressler KJ, 2021. The renin-angiotensin system in PTSD: a replication and extension. Neuropsychopharmacology 46, 750–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selten JP, van der Ven E, Rutten BP, Cantor-Graae E, 2013. The social defeat hypothesis of schizophrenia: an update. Schizophr Bull 39, 1180–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selye H, 1950a. The physiology and pathology of exposure to stress : a treatise based on the concepts of the general-adaptation-syndrome and the diseases of adaptation. Acta, Montreal. [Google Scholar]
- Selye H, 1950b. Stress and the general adaptation syndrome. Br Med J 1, 1383–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selye H, 1976. The stress concept. Can Med Assoc J 115, 718. [PMC free article] [PubMed] [Google Scholar]
- Selye H, 1998. A syndrome produced by diverse nocuous agents. 1936. J Neuropsychiatry Clin Neurosci 10, 230–231. [DOI] [PubMed] [Google Scholar]
- Senchenkova EY, Russell J, Yildirim A, Granger DN, Gavins FNE, 2019. Novel Role of T Cells and IL-6 (Interleukin-6) in Angiotensin II-Induced Microvascular Dysfunction. Hypertension 73, 829–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo DO, Lee S, Rivier C, 2003. Role of specific adrenergic receptors in mediating the adrenocorticotropic hormone response to increased nitric oxide levels. J Neuroendocrinol 15, 530–537. [DOI] [PubMed] [Google Scholar]
- Shabani A, Masoumian S, Zamirinejad S, Hejri M, Pirmorad T, Yaghmaeezadeh H, 2021. Psychometric properties of Structured Clinical Interview for DSM-5 Disorders-Clinician Version (SCID-5-CV). Brain Behav 11, e01894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankman SA, Funkhouser CJ, Klein DN, Davila J, Lerner D, Hee D, 2018. Reliability and validity of severity dimensions of psychopathology assessed using the Structured Clinical Interview for DSM-5 (SCID). Int J Methods Psychiatr Res 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilton AL, Laycock R, Crewther SG, 2017. The Maastricht Acute Stress Test (MAST): Physiological and Subjective Responses in Anticipation, and Post-stress. Front Psychol 8, 567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sial OK, Warren BL, Alcantara LF, Parise EM, Bolanos-Guzman CA, 2016. Vicarious social defeat stress: Bridging the gap between physical and emotional stress. J Neurosci Methods 258, 94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RA, Duker EM, Pahnke U, Wuttke W, 1987. Stress-induced changes in cholecystokinin and substance P concentrations in discrete regions of the rat hypothalamus. Neuroendocrinology 46, 75–81. [DOI] [PubMed] [Google Scholar]
- Singh VK, Seed TM, 2021. How necessary are animal models for modern drug discovery? Expert Opin Drug Discov 16, 1391–1397. [DOI] [PubMed] [Google Scholar]
- Skov J, Persson F, Frokiaer J, Christiansen JS, 2014. Tissue Renin-Angiotensin systems: a unifying hypothesis of metabolic disease. Front Endocrinol (Lausanne) 5, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeets T, Cornelisse S, Quaedflieg CW, Meyer T, Jelicic M, Merckelbach H, 2012. Introducing the Maastricht Acute Stress Test (MAST): a quick and non-invasive approach to elicit robust autonomic and glucocorticoid stress responses. Psychoneuroendocrinology 37, 1998–2008. [DOI] [PubMed] [Google Scholar]
- Smith SM, Vale WW, 2006. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci 8, 383–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth J, Ockenfels MC, Porter L, Kirschbaum C, Hellhammer DH, Stone AA, 1998. Stressors and mood measured on a momentary basis are associated with salivary cortisol secretion. Psychoneuroendocrinology 23, 353–370. [DOI] [PubMed] [Google Scholar]
- Somvanshi PR, Mellon SH, Yehuda R, Flory JD, Makotkine I, Bierer L, Marmar C, Jett M, Doyle FJ 3rd, 2020. Role of enhanced glucocorticoid receptor sensitivity in inflammation in PTSD: insights from computational model for circadian-neuroendocrine-immune interactions. Am J Physiol Endocrinol Metab 319, E48–E66. [DOI] [PubMed] [Google Scholar]
- Song Z, Albers HE, 2018. Cross-talk among oxytocin and arginine-vasopressin receptors: Relevance for basic and clinical studies of the brain and periphery. Front Neuroendocrinol 51, 14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto-Rivera CL, Majzoub, Joseph A, 2017. Chapter 3 - Adrenocorticotrophin. Academic Press. [Google Scholar]
- Spanakis EK, Wand GS, Ji N, Golden SH, 2016. Association of HPA axis hormones with copeptin after psychological stress differs by sex. Psychoneuroendocrinology 63, 254–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaniol J, Davidson PS, Kim AS, Han H, Moscovitch M, Grady CL, 2009. Event-related fMRI studies of episodic encoding and retrieval: meta-analyses using activation likelihood estimation. Neuropsychologia 47, 1765–1779. [DOI] [PubMed] [Google Scholar]
- Stanton LM, Price AJ, Manning EE, 2023. Hypothalamic corticotrophin releasing hormone neurons in stress-induced psychopathology: Revaluation of synaptic contributions. J Neuroendocrinol 35, e13268. [DOI] [PubMed] [Google Scholar]
- Steensberg A, Toft AD, Schjerling P, Halkjaer-Kristensen J, Pedersen BK, 2001. Plasma interleukin-6 during strenuous exercise: role of epinephrine. Am J Physiol Cell Physiol 281, C1001–1004. [DOI] [PubMed] [Google Scholar]
- Stein DJ, Scott K, Haro Abad JM, Aguilar-Gaxiola S, Alonso J, Angermeyer M, Demytteneare K, de Girolamo G, Iwata N, Posada-Villa J, Kovess V, Lara C, Ormel J, Kessler RC, Von Korff M, 2010. Early childhood adversity and later hypertension: data from the World Mental Health Survey. Ann Clin Psychiatry 22, 19–28. [PMC free article] [PubMed] [Google Scholar]
- Steiner JE, Glaser D, Hawilo ME, Berridge KC, 2001. Comparative expression of hedonic impact: affective reactions to taste by human infants and other primates. Neurosci Biobehav Rev 25, 53–74. [DOI] [PubMed] [Google Scholar]
- Stojiljkovic AS, Cupic Z, Macesic S, Ivanovic-Sasic A, Kolar-Anic L, 2022. Influence of arginine vasopressin on the ultradian dynamics of Hypothalamic-Pituitary-Adrenal axis. Front Endocrinol (Lausanne) 13, 976323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stremple JF, Ellison EH, Carey LC, 1966. Osmolar diuresis: success and/or failure. A collective review. Surgery 60, 924–937. [PubMed] [Google Scholar]
- Suffredini AF, Hochstein HD, McMahon FG, 1999. Dose-related inflammatory effects of intravenous endotoxin in humans: evaluation of a new clinical lot of Escherichia coli O:113 endotoxin. J Infect Dis 179, 1278–1282. [DOI] [PubMed] [Google Scholar]
- SVHC, S.V.s.H.C., 2023. Dobutamine stress echocardiography. St. Vincent’s Heart Center. [Google Scholar]
- Swaab DF, Bao AM, Lucassen PJ, 2005. The stress system in the human brain in depression and neurodegeneration. Ageing Res Rev 4, 141–194. [DOI] [PubMed] [Google Scholar]
- Swann AC, Lijffijt M, Lane SD, Cox B, Steinberg JL, Moeller FG, 2013. Norepinephrine and impulsivity: effects of acute yohimbine. Psychopharmacology (Berl) 229, 83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE, 1980. Paraventricular nucleus: a site for the integration of neuroendocrine and autonomic mechanisms. Neuroendocrinology 31, 410–417. [DOI] [PubMed] [Google Scholar]
- Swedo SE, Leonard HL, Garvey M, Mittleman B, Allen AJ, Perlmutter S, Lougee L, Dow S, Zamkoff J, Dubbert BK, 1998. Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections: clinical description of the first 50 cases. Am J Psychiatry 155, 264–271. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Lehrner A, Yehuda R, 2018. Glucocorticoids and Hippocampal Structure and Function in PTSD. Harv Rev Psychiatry 26, 142–157. [DOI] [PubMed] [Google Scholar]
- Tabak BA, Rosenfield D, Sunahara CS, Alvi T, Szeto A, Mendez AJ, 2022. Social anxiety is associated with greater peripheral oxytocin reactivity to psychosocial stress. Psychoneuroendocrinology 140, 105712. [DOI] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Favilla S, Salvetti A, 1992. Adenosine activates a vascular renin-angiotensin system in hypertensive subjects. Hypertension 19, 672–675. [DOI] [PubMed] [Google Scholar]
- Takahashi A, Ishimaru H, Ikarashi Y, Kishi E, Maruyama Y, 1994. Hypothalamic cholinergic and noradrenergic neurons in hyperglycemia induced by 2-deoxyglucose. Brain Res 665, 13–17. [DOI] [PubMed] [Google Scholar]
- Takai N, Yamaguchi M, Aragaki T, Eto K, Uchihashi K, Nishikawa Y, 2004. Effect of psychological stress on the salivary cortisol and amylase levels in healthy young adults. Arch Oral Biol 49, 963–968. [DOI] [PubMed] [Google Scholar]
- Takayanagi Y, Onaka T, 2021. Roles of Oxytocin in Stress Responses, Allostasis and Resilience. Int J Mol Sci 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam SW, Worcel M, Wyllie M, 2001. Yohimbine: a clinical review. Pharmacol Ther 91, 215–243. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Yoshida M, Emoto H, Ishii H, 2000. Noradrenaline systems in the hypothalamus, amygdala and locus coeruleus are involved in the provocation of anxiety: basic studies. Eur J Pharmacol 405, 397–406. [DOI] [PubMed] [Google Scholar]
- Taylor BK, Westlund KN, 2017. The noradrenergic locus coeruleus as a chronic pain generator. J Neurosci Res 95, 1336–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor PM, 1998. Endocrine and metabolic responses in sheep during halothane and pentobarbitone anaesthesia with dobutamine infusion. J Vet Pharmacol Ther 21, 62–68. [DOI] [PubMed] [Google Scholar]
- Tennant F, 2013. The physiologic effects of pain on the endocrine system. Pain Ther 2, 75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennant F, Hermann L, 2002. Normalization of serum cortisol concentration with opioid treatment of severe chronic pain. Pain Med 3, 132–134. [DOI] [PubMed] [Google Scholar]
- Teo CH, Wong ACH, Sivakumaran RN, Parhar I, Soga T, 2023. Gender Differences in Cortisol and Cortisol Receptors in Depression: A Narrative Review. Int J Mol Sci 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thau L, Reddy V, Singh P, 2023. Anatomy, Central Nervous System, StatPearls, Treasure Island (FL). [PubMed] [Google Scholar]
- Thornorarinsdottir H, Faurholt-Jepsen M, Ullum H, Frost M, Bardram JE, Kessing LV, 2019. The Validity of Daily Self-Assessed Perceived Stress Measured Using Smartphones in Healthy Individuals: Cohort Study. JMIR Mhealth Uhealth 7, e13418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, Ma L, Kaarela T, Li Z, 2012. Neuroimmune crosstalk in the central nervous system and its significance for neurological diseases. J Neuroinflammation 9, 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian R, Hou G, Li D, Yuan TF, 2014. A possible change process of inflammatory cytokines in the prolonged chronic stress and its ultimate implications for health. ScientificWorldJournal 2014, 780616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmermans PB, Wong PC, Chiu AT, Herblin WF, Benfield P, Carini DJ, Lee RJ, Wexler RR, Saye JA, Smith RD, 1993. Angiotensin II receptors and angiotensin II receptor antagonists. Pharmacol Rev 45, 205–251. [PubMed] [Google Scholar]
- Tochikubo O, Ikeda A, Miyajima E, Ishii M, 1996. Effects of insufficient sleep on blood pressure monitored by a new multibiomedical recorder. Hypertension 27, 1318–1324. [DOI] [PubMed] [Google Scholar]
- Torrico TJ, Munakomi S, 2023. Neuroanatomy, Thalamus, StatPearls, Treasure Island (FL). [PubMed] [Google Scholar]
- Toyoda A, 2017. Social defeat models in animal science: What we have learned from rodent models. Anim Sci J 88, 944–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triedman JK, Cohen RJ, Saul JP, 1993. Mild hypovolemic stress alters autonomic modulation of heart rate. Hypertension 21, 236–247. [DOI] [PubMed] [Google Scholar]
- Tryon MS, Stanhope KL, Epel ES, Mason AE, Brown R, Medici V, Havel PJ, Laugero KD, 2015. Excessive Sugar Consumption May Be a Difficult Habit to Break: A View From the Brain and Body. J Clin Endocrinol Metab 100, 2239–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai CS, Mitton KP, Johnson BF, 1989. Acetate assimilation by the fission yeast, Schizosaccharomyces pombe. Biochem Cell Biol 67, 464–467. [DOI] [PubMed] [Google Scholar]
- Tsigos C, Chrousos GP, 1994. Physiology of the hypothalamic-pituitary-adrenal axis in health and dysregulation in psychiatric and autoimmune disorders. Endocrinol Metab Clin North Am 23, 451–466. [PubMed] [Google Scholar]
- Tsigos C, Chrousos GP, 2002. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Res 53, 865–871. [DOI] [PubMed] [Google Scholar]
- Tsigos C, Kyrou I, Kassi E, Chrousos GP, 2000. Stress: Endocrine Physiology and Pathophysiology, in: Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya K, Dungan K, Hofland J, Kalra S, Kaltsas G, Kapoor N, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrere B, Levy M, McGee EA, McLachlan R, New M, Purnell J, Sahay R, Shah AS, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP (Eds.), Endotext, South Dartmouth (MA). [PubMed] [Google Scholar]
- Tsigos C, Reed P, Weinkove C, White A, Young RJ, 1993. Plasma norepinephrine in sensory diabetic polyneuropathy. Diabetes Care 16, 722–727. [DOI] [PubMed] [Google Scholar]
- Tsuji B, Hayashi K, Kondo N, Nishiyasu T, 2016. Characteristics of hyperthermia-induced hyperventilation in humans. Temperature (Austin) 3, 146–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker P, Czapla CS, 2021. Post-COVID Stress Disorder: Another Emerging Consequence of the Global Pandemic. Psychiatric Times 38. [Google Scholar]
- Tulen JH, Moleman P, van Steenis HG, Boomsma F, 1989. Characterization of stress reactions to the Stroop Color Word Test. Pharmacol Biochem Behav 32, 9–15. [DOI] [PubMed] [Google Scholar]
- Ullian ME, 1999. The role of corticosteriods in the regulation of vascular tone. Cardiovasc Res 41, 55–64. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Engeland WC, 2002. Adrenal splanchnic innervation modulates adrenal cortical responses to dehydration stress in rats. Neuroendocrinology 76, 79–92. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Figueiredo HF, Ostrander MM, Choi DC, Engeland WC, Herman JP, 2006. Chronic stress induces adrenal hyperplasia and hypertrophy in a subregion-specific manner. Am J Physiol Endocrinol Metab 291, E965–973. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP, 2009. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci 10, 397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umhau JC, Schwandt ML, Usala J, Geyer C, Singley E, George DT, Heilig M, 2011. Pharmacologically induced alcohol craving in treatment seeking alcoholics correlates with alcoholism severity, but is insensitive to acamprosate. Neuropsychopharmacology 36, 1178–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uvnas Moberg K, Handlin L, Kendall-Tackett K, Petersson M, 2019. Oxytocin is a principal hormone that exerts part of its effects by active fragments. Med Hypotheses 133, 109394. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Van Bockstaele E, 2008. Convergent regulation of locus coeruleus activity as an adaptive response to stress. Eur J Pharmacol 583, 194–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Calker D, Biber K, Domschke K, Serchov T, 2019. The role of adenosine receptors in mood and anxiety disorders. J Neurochem 151, 11–27. [DOI] [PubMed] [Google Scholar]
- van der Molen HF, Nieuwenhuijsen K, Frings-Dresen MHW, de Groene G, 2020. Work-related psychosocial risk factors for stress-related mental disorders: an updated systematic review and meta-analysis. BMJ Open 10, e034849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Poll T, Coyle SM, Barbosa K, Braxton CC, Lowry SF, 1996a. Epinephrine inhibits tumor necrosis factor-alpha and potentiates interleukin 10 production during human endotoxemia. J Clin Invest 97, 713–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Poll T, Coyle SM, Moldawer LL, Lowry SF, 1996b. Changes in endotoxin-induced cytokine production by whole blood after in vivo exposure of normal humans to endotoxin. J Infect Dis 174, 1356–1360. [DOI] [PubMed] [Google Scholar]
- Van der Poll T, Lowry SF, 1997a. Epinephrine inhibits endotoxin-induced IL-1 beta production: roles of tumor necrosis factor-alpha and IL-10. Am J Physiol 273, R1885–1890. [DOI] [PubMed] [Google Scholar]
- van der Poll T, Lowry SF, 1997b. Lipopolysaccharide-induced interleukin 8 production by human whole blood is enhanced by epinephrine and inhibited by hydrocortisone. Infect Immun 65, 2378–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ruitenbeek P, Quaedflieg CW, Hernaus D, Hartogsveld B, Smeets T, 2021. Dopaminergic and noradrenergic modulation of stress-induced alterations in brain activation associated with goal-directed behaviour. J Psychopharmacol 35, 1449–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Winkel R, Stefanis NC, Myin-Germeys I, 2008. Psychosocial stress and psychosis. A review of the neurobiological mechanisms and the evidence for gene-stress interaction. Schizophr Bull 34, 1095–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varghese FP, Brown ES, 2001. The Hypothalamic-Pituitary-Adrenal Axis in Major Depressive Disorder: A Brief Primer for Primary Care Physicians. Prim Care Companion J Clin Psychiatry 3, 151–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasa RA, Pine DS, Masten CL, Vythilingam M, Collin C, Charney DS, Neumeister A, Mogg K, Bradley BP, Bruck M, Monk CS, 2009. Effects of yohimbine and hydrocortisone on panic symptoms, autonomic responses, and attention to threat in healthy adults. Psychopharmacology (Berl) 204, 445–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazan R, Filcikova D, Mravec B, 2017. Effect of the Stroop test performed in supine position on the heart rate variability in both genders. Auton Neurosci 208, 156–160. [DOI] [PubMed] [Google Scholar]
- Velasco M, Gomez J, Blanco M, Rodriguez I, 1997. The cold pressor test: pharmacological and therapeutic aspects. Am J Ther 4, 34–38. [DOI] [PubMed] [Google Scholar]
- Velez-Roa S, Renard M, Degaute JP, van de Borne P, 2003. Peripheral sympathetic control during dobutamine infusion: effects of aging and heart failure. J Am Coll Cardiol 42, 1605–1610. [DOI] [PubMed] [Google Scholar]
- Venniro M, Banks ML, Heilig M, Epstein DH, Shaham Y, 2020. Improving translation of animal models of addiction and relapse by reverse translation. Nat Rev Neurosci 21, 625–643. [DOI] [PubMed] [Google Scholar]
- Videlock EJ, Peleg T, Segman R, Yehuda R, Pitman RK, Shalev AY, 2008. Stress hormones and post-traumatic stress disorder in civilian trauma victims: a longitudinal study. Part II: the adrenergic response. Int J Neuropsychopharmacol 11, 373–380. [DOI] [PubMed] [Google Scholar]
- Vinstrup J, Jakobsen MD, Andersen LL, 2020. Perceived Stress and Low-Back Pain Among Healthcare Workers: A Multi-Center Prospective Cohort Study. Front Public Health 8, 297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitiello A, Ferrara F, 2020. Correlation between renin-angiotensin system and Severe Acute Respiratory Syndrome Coronavirus 2 infection: What do we know? Eur J Pharmacol 883, 173373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner S, Mohr-Kahaly S, Nixdorff U, Kolsch B, Menzel T, Wittlich N, Meinert R, Meyer J, 1996. [Prospective study of subjective stress caused by dobutamine stress echocardiography--effect on diagnostic accuracy]. Z Kardiol 85, 588–595. [PubMed] [Google Scholar]
- Wang LI, Liu F, Luo Y, Zhu L, Li G, 2015. Effect of acute heat stress on adrenocorticotropic hormone, cortisol, interleukin-2, interleukin-12 and apoptosis gene expression in rats. Biomed Rep 3, 425–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers FW, Bovin MJ, Lee DJ, Sloan DM, Schnurr PP, Kaloupek DG, Keane TM, Marx BP, 2018. The Clinician-Administered PTSD Scale for DSM-5 (CAPS-5): Development and initial psychometric evaluation in military veterans. Psychol Assess 30, 383–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WebMD, 2023. Yohimbe - Uses, Side Effects, and More, WebMD. [Google Scholar]
- Welch KA, Carson AJ, 2018. When psychiatric symptoms reflect medical conditions. Clin Med (Lond) 18, 80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westen D, Rosenthal R, 2003. Quantifying construct validity: two simple measures. J Pers Soc Psychol 84, 608–618. [DOI] [PubMed] [Google Scholar]
- Whiteman PJ, Macias-Konstantopoulos WL, Relan P, Knopov A, Ranney ML, Riviello RJ, 2023. Violence and Abuse: A Pandemic Within a Pandemic. West J Emerg Med 24, 743–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitworth JA, 1994. Studies on the mechanisms of glucocorticoid hypertension in humans. Blood Press 3, 24–32. [DOI] [PubMed] [Google Scholar]
- Wiedermann FJ, Watzinger K, Stichlberger M, Joannidis M, Kaehler C, Lederer W, 2018. Effects of Arginine Vasopressin on Migration and Respiratory Burst Activity in Human Leukocytes. Open Med (Wars) 13, 122–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson JB, Jaffee MS, Jorge RE, 2021. Posttraumatic Stress Disorder and Anxiety-Related Conditions. Continuum (Minneap Minn) 27, 1738–1763. [DOI] [PubMed] [Google Scholar]
- Willner P, 1984. The validity of animal models of depression. Psychopharmacology (Berl) 83, 1–16. [DOI] [PubMed] [Google Scholar]
- Wilson TE, Klabunde RE, Monahan KD, 2014. Using thermal stress to model aspects of disease states. J Therm Biol 43, 24–32. [DOI] [PubMed] [Google Scholar]
- Wippert PM, Puerto Valencia L, Driesslein D, 2022. Stress and Pain. Predictive (Neuro)Pattern Identification for Chronic Back Pain: A Longitudinal Observational Study. Front Med (Lausanne) 9, 828954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise PM, Nattress L, Flammer LJ, Beauchamp GK, 2016. Reduced dietary intake of simple sugars alters perceived sweet taste intensity but not perceived pleasantness. Am J Clin Nutr 103, 50–60. [DOI] [PubMed] [Google Scholar]
- Wise RA, 2002. Brain reward circuitry: insights from unsensed incentives. Neuron 36, 229–240. [DOI] [PubMed] [Google Scholar]
- Wotjak CT, Kubota M, Liebsch G, Montkowski A, Holsboer F, Neumann I, Landgraf R, 1996. Release of vasopressin within the rat paraventricular nucleus in response to emotional stress: a novel mechanism of regulating adrenocorticotropic hormone secretion? J Neurosci 16, 7725–7732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtman RJ, 2002. Stress and the adrenocortical control of epinephrine synthesis. Metabolism 51, 11–14. [DOI] [PubMed] [Google Scholar]
- Wurtman RJ, Axelrod J, 1966. Control of enzymatic synthesis of adrenaline in the adrenal medulla by adrenal cortical steroids. J Biol Chem 241, 2301–2305. [PubMed] [Google Scholar]
- Xiao D, Huang X, Pearce WJ, Longo LD, Zhang L, 2003. Effect of cortisol on norepinephrine-mediated contractions in ovine uterine arteries. Am J Physiol Heart Circ Physiol 284, H1142–1151. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Iwamoto M, Inoue M, Harada N, 2007. Evaluation of the effect of heat exposure on the autonomic nervous system by heart rate variability and urinary catecholamines. J Occup Health 49, 199–204. [DOI] [PubMed] [Google Scholar]
- Yang YL, Gordon CJ, 2002. Possible role of vasopressin in the thermoregulatory response to chlorpyrifos in the rat. Pharmacol Toxicol 90, 311–316. [DOI] [PubMed] [Google Scholar]
- Yimcharoen S, Zhang S, Kaolawanich Y, Tanapibunpon P, Krittayaphong R, 2020. Clinical assessment of adenosine stress and rest cardiac magnetic resonance T1 mapping for detecting ischemic and infarcted myocardium. Sci Rep 10, 14727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young Kuchenbecker S, Pressman SD, Celniker J, Grewen KM, Sumida KD, Jonathan N, Everett B, Slavich GM, 2021. Oxytocin, cortisol, and cognitive control during acute and naturalistic stress. Stress 24, 370–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Liu S, Bai X, Gao Y, Liu G, Wang X, Liu D, Li T, Hao A, Wang Z, 2016. Oxytocin inhibits lipopolysaccharide-induced inflammation in microglial cells and attenuates microglial activation in lipopolysaccharide-treated mice. J Neuroinflammation 13, 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JM, An J, 2007. Cytokines, inflammation, and pain. Int Anesthesiol Clin 45, 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Brinton RD, 2004. Suppression of proinflammatory cytokines interleukin-1beta and tumor necrosis factor-alpha in astrocytes by a V1 vasopressin receptor agonist: a cAMP response element-binding protein-dependent mechanism. J Neurosci 24, 2226–2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler DR, Herman JP, 2002. Neurocircuitry of stress integration: anatomical pathways regulating the hypothalamo-pituitary-adrenocortical axis of the rat. Integr Comp Biol 42, 541–551. [DOI] [PubMed] [Google Scholar]
- Zimbardo PG, 1973. On the ethics of intervention in human psychological research: with special reference to the Stanford Prison Experiment. Cognition 2, 243–256. [DOI] [PubMed] [Google Scholar]
- Zorn JV, Schur RR, Boks MP, Kahn RS, Joels M, Vinkers CH, 2017. Cortisol stress reactivity across psychiatric disorders: A systematic review and meta-analysis. Psychoneuroendocrinology 77, 25–36. [DOI] [PubMed] [Google Scholar]