Abstract
Linoleic acid (LA), an n-6 polyunsaturated fatty acid (PUFA), is obtained from the maternal diet during pregnancy, and is essential for normal fetal growth and development. A maternal high-LA (HLA) diet alters maternal and offspring fatty acids, maternal leptin and male/female ratio at embryonic (E) day 20 (E20). We investigated the effects of an HLA diet on embryonic offspring renal branching morphogenesis, leptin signalling, megalin signalling and angiogenesis gene expression. Female Wistar Kyoto rats were fed low-LA (LLA; 1.44% energy from LA) or high-LA (HLA; 6.21% energy from LA) diets during pregnancy and gestation/lactation. Offspring were sacrificed and mRNA from kidneys was analysed by real-time PCR. Maternal HLA decreased the targets involved in branching morphogenesis Ret and Gdnf in offspring, independent of sex. Furthermore, downstream targets of megalin, namely mTOR, Akt3 and Prkab2, were reduced in offspring from mothers consuming an HLA diet, independent of sex. There was a trend of an increase in the branching morphogenesis target Gfra1 in females (p = 0.0517). These findings suggest that an HLA diet during pregnancy may lead to altered renal function in offspring. Future research should investigate the effects an HLA diet has on offspring kidney function in adolescence and adulthood.
Keywords: linoleic acid, maternal, offspring, sex specific, kidney
1. Introduction
In Westernised societies, we are consuming more plant-based fats, with an abundance of the omega 6 (n—6) polyunsaturated fatty acid (PUFA) linoleic acid (LA; 18:2n—6; cis, cis-9,12-octadecadienoic acid) [1]. Dietary availability of LA has increased dramatically in the last 50 years [2]. LA is an essential fatty acid which is required by the body for functions such as modulating cell signalling and expression of genes, and it also has a role in inflammation [3]. LA and the omega 3 (n—3) fatty acid (FA) α—linoleic acid (ALA) are metabolised by the same enzymes [4]. A metabolite of LA is arachidonic acid (AA), which plays an integral role in the inflammatory pathway [4]. In pregnant women, the elevated intake of n—6 FAs in the Western diet is reflected in a maldistributed fatty acid profile [5]. As LA can be transported by the placenta to the foetus during development [6], a maternal elevated LA diet may impact fetal development and offsprings’ developmental outcomes.
Previous research from our group in a rodent model demonstrated that a maternal high-LA diet during pregnancy alters male/female sex ratio, circulating leptin, maternal and offspring circulating fatty acids [7] and placental FA and FA transporters [8]. Notably, there was no change in rodent offsprings’ whole organ weight [7]; however, in rodent offspring, the diet led to sex-specific cardiovascular changes [9] and hepatic changes in adolescence [10] and adulthood [11]. Importantly, leptin is critical for normal organ development [12], and adequate leptin concentrations are required for kidney development in rodents [13]. At this time, the effects of a maternal high-LA diet on offspring renal development are unknown. This is important for life-long health, as importantly, reduced nephron endowment in humans increases the risk of developing a number of diseases in adulthood [14].
Kidney development is a complex process, with a number of key targets involved in nephrogenesis. Genes regulating renal branching morphogenesis, renal growth, cellular proliferation and apoptosis are altered in developing rat kidneys due to an adverse maternal environment [14,15,16,17]. Branching morphogenesis is a process that assists in establishing the collecting ducts to the adult organ, and drives organ expansion via peripheral interactions with nephron progenitor cells [18]. Angiogenesis, which involves the development of blood vessels from the pre-existing vascular system, is crucial for normal adult kidney development. Factors which regulate development can be varied, but research has demonstrated that adipokine leptin in rodents plays a critical role in development [13]. Most of our understanding concerning leptin’s developmental influence [19] has focused on neuronal systems due to leptin’s ability to regulate appetite [20]. However, antagonism of leptin in the postnatal rodent signalling window demonstrated a reduction in the maturation of the kidneys [12]. The mechanism for this is unknown. However, leptin can bind to both the leptin receptor and megalin in the kidney [21].
Developmental programming of disease risk due to an imbalanced maternal diet during pregnancy has led to the identification of sex-specific effects in offspring outcomes [11,22,23,24,25,26]. Therefore, in this study, we aimed to investigate the effects of a maternal high-LA (HLA) diet on targets responsible for renal development in embryonic rat offspring. As developmental programming is often sex-specific [11,22,23,24,25,26], we analysed both male and female embryonic offspring independently. It was hypothesised that the kidneys of offspring from mothers consuming an HLA diet would have altered branching morphogenesis, angiogenesis and leptin and megalin signalling compared to those from offspring of mothers consuming an LLA diet, with males being more significantly affected than females.
2. Results
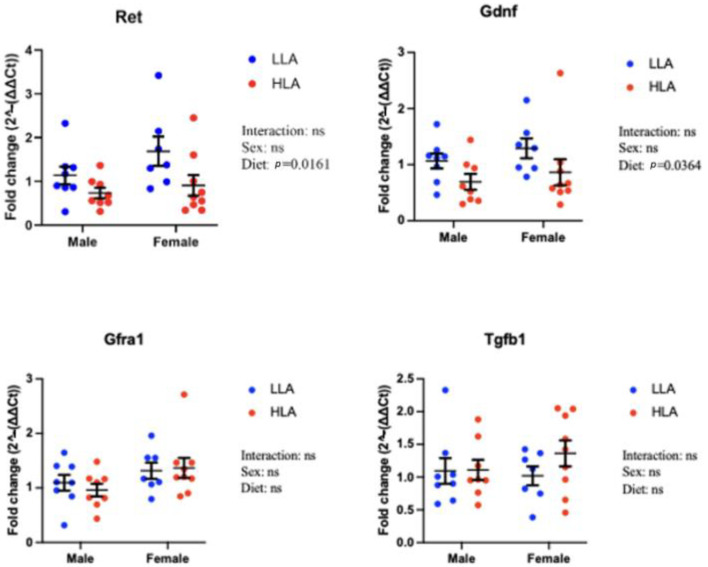
2.1. Effect of Maternal HLA Diet on Renal Genes Involved in Branching Morphogenesis
The organ and body weights from the E20 cohort have previously been published [7]. These data demonstrated that for both males and females, and for both left and right kidneys, a maternal HLA diet did not alter kidney weight or body weight [7]. To extend this study, we investigated renal genes involved in branching morphogenesis (Figure 1). A maternal HLA diet decreased Ret (Figure 1, n = 6–8, p = 0.0161) and Gdnf (Figure 1, n = 6–8, p = 0.0364). There was a trend towards significance for Gfra1, with an increase in females (Figure 1, n = 6–8, p = 0.0517). Tgfb1 was unaltered in response to a maternal HLA diet (Figure 1).
Figure 1.
Effect of maternal diet high in linoleic acid on branching morphogenesis genes in the kidneys of embryonic offspring. Data are presented as mean ± standard error of the mean (SEM). Two-way ANOVA was performed for statistical analysis with maternal diet and sex as two factors. n = 6–8. LLA: low linoleic acid; HLA: high linoleic acid. ns = not significant.
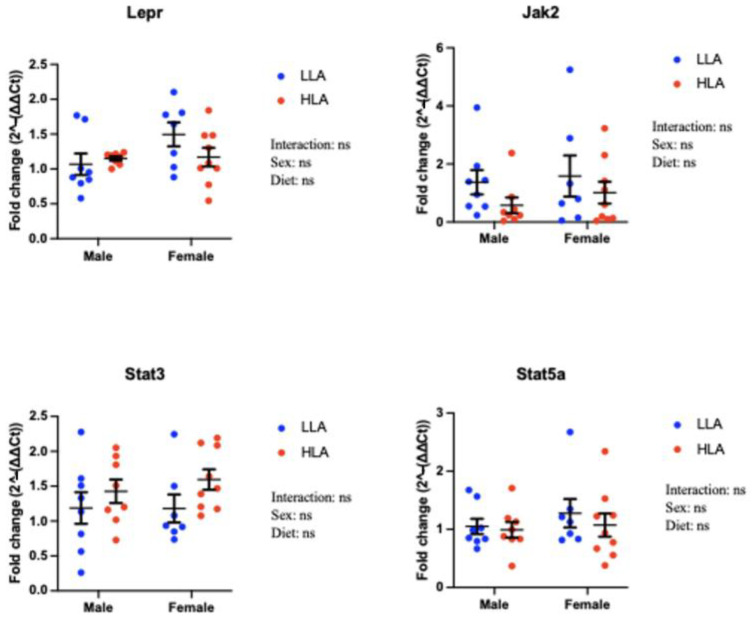
2.2. Effect of Maternal HLA Diet on Renal Genes Involved in Leptin Signalling in Embryonic Offspring
There were no changes to Lepr, Jak2, Stat3 or Stat5a in response to a maternal HLA diet (Figure 2, n = 6–8).
Figure 2.
Effect of maternal diet high in linoleic acid on leptin signalling genes in the kidneys of embryonic offspring. Data are presented as mean ± standard error of the mean (SEM). Two-way ANOVA was performed for statistical analysis with maternal diet and sex as two factors. n = 6–8. LLA: low linoleic acid; HLA: high linoleic acid. ns = not significant.
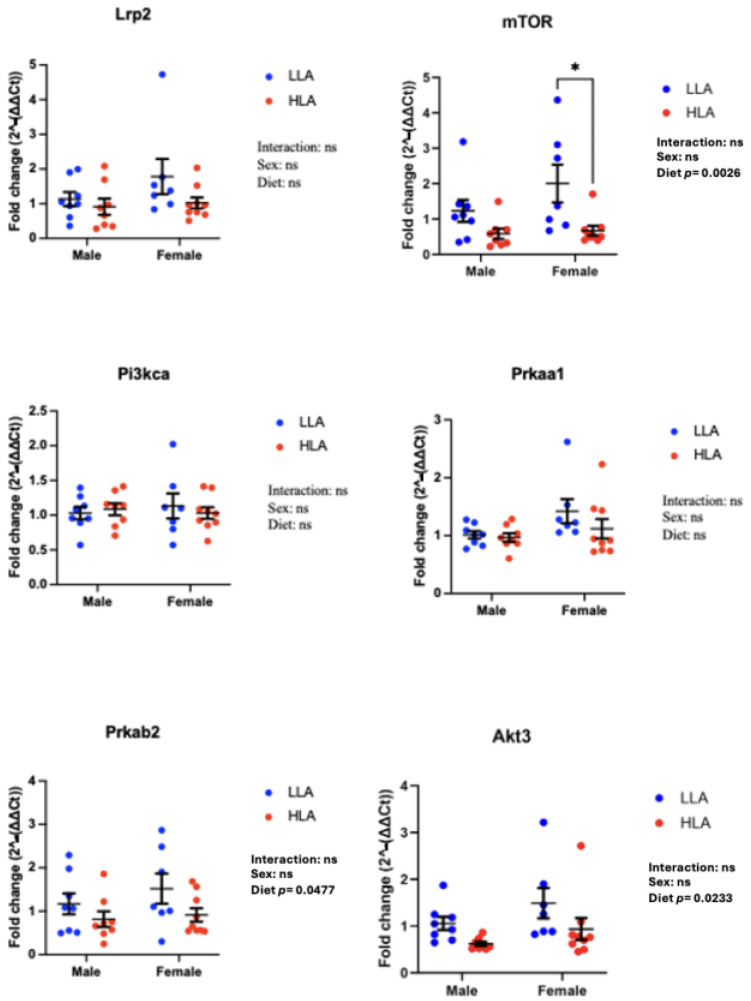
2.3. Effect of Maternal HLA Diet on Renal Genes Involved in Megalin Signalling in Embryonic Offspring
A maternal HLA diet decreased mTOR (Figure 3, n = 6–8, p = 0.0026), Prkab2 (Figure 3, n = 6–8, p = 0.0477) and Akt3 (Figure 3, n = 6–8, p = 0.0233). Lrp2 (megalin), Pi3kca and Prkaa1 were unaltered in response to a maternal HLA diet (Figure 3). Post hoc analysis indicated there was a significant decrease in mTOR between females from mothers consuming LLA and those from mothers consuming HLA (Figure 3).
Figure 3.
Effect of maternal diet high in linoleic acid on megalin signalling genes in the kidneys of embryonic offspring. Data are presented as mean ± standard error of the mean (SEM). Two-way ANOVA was performed for statistical analysis with maternal diet and sex as two factors. n = 6–8. LLA: low linoleic acid; HLA: high linoleic acid. Where post hoc analysis identified a difference, differences across the groups are denoted by an asterisk (* p < 0.05). ns = not significant.
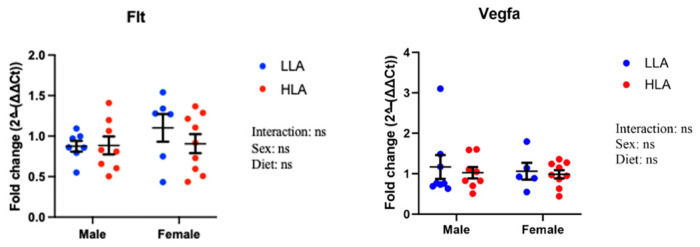
2.4. Effect of Maternal HLA Diet on Angiogenesis Genes in Embryonic Kidneys
Flt and Vegfa were unaltered in response to a maternal HLA diet (Figure 4).
Figure 4.
Effect of maternal diet high in linoleic acid on angiogenesis genes in the kidneys of embryonic offspring. Data are presented as mean ± standard error of the mean (SEM). Two-way ANOVA was performed for statistical analysis with maternal diet and postnatal diet as two factors. n = 6–8. LLA: low linoleic acid; HLA: high linoleic acid. ns = not significant.
3. Discussion
In this study, we have demonstrated that a maternal HLA diet reduces the expression of genes responsible for branching morphogenesis (Ret, Gdnf) and megalin-mediated signalling (mTOR, Akt3 and Prkab2). The maternal environment, particularly nutrition during pregnancy, can have significant effects on fetal growth and development [27]. Our previous research demonstrated that there are early life consequences of a maternal HLA diet [7]. Specifically, we demonstrated that leptin is reduced in mothers consuming an HLA diet. Leptin is critical for development [19]. Previous research has demonstrated that blunting the leptin postnatal peak during the first week of life in rats (corresponding to the completion of nephrogenesis) reduces the number of glomeruli [12]. However, the molecular mechanism for this was unknown. We have previously demonstrated that a maternal HLA diet reduces maternal serum leptin and alters dietary FAs [7]. To add to this, we have demonstrated that this maternal HLA diet impacts targets responsible for branching morphogenesis and mTOR/AKT signalling. This may contribute to the altered nephrogenesis observed when leptin is antagonised [12].
The change in gene expression is surprising, as in our model of a maternal HLA diet, kidney weight was unaltered at E20 [7]. Notably, kidney weight changes were also not observed at adolescence [9] or adulthood [11]. Despite this, a key finding in this study was the decreased expression of genes involved in branching morphogenesis, one of the initial stages of nephrogenesis, in the offspring of the HLA diet group. These genes, Ret and Gdnf, play an important role in the development of the kidneys [28]. Gdnf binds to Ret, activating it and allowing the process of proliferation and branching of the ureteric bud, an integral step in forming the kidney [28]. The decreased expression of Gdnf found in the offspring from mothers consuming an HLA diet could potentially decrease the number of nephrons formed during the nephrogenic process. Strong correlations between the early ureteric branching extent and nephron endowment have previously been found [29]. Specifically, a rodent model of food restriction in the mother resulted in decreased Gdnf mRNA and protein expression levels in the absence of changes to Ret [30]. This study is supported by a similar model where mothers consumed 50% fewer calories, which resulted in a reduction in Gdnf for both mRNA and protein [31]. The reduction in Gdnf and Ret has the potential to reduce the nephron number and therefore could impact kidney function later in adulthood [30]. Importantly, with the removal of Gdnf/Ret during kidney development in mice, there were significant branching abnormalities [32]. In these models of maternal food restriction, the maternal body weight and thus leptin were reduced, suggesting that leptin may regulate branching morphogenesis via a Gdnf/Ret pathway. Of interest, a previous study has shown that there are sex-specific differences in Gdnf in mice [33], which was not observed in our study in rats. Furthermore, in this study in mice, a maternal low-protein diet did not alter Gdnf mRNA [33]. Thus, the reduction in Gdnf in this study suggests that reduced leptin and/or altered fatty acids may impact branching morphogenesis, independently of sex.
Another novel outcome from this study was that in offspring from the maternal HLA diet group, there was decreased expression of downstream signalling targets of leptin in the megalin signalling pathway, namely mTOR, Akt3 and Prkab2. Megalin is an endocytic receptor, highly expressed in the renal proximal tubule, and plays a vital role in the reabsorption of macromolecules filtered in the kidneys [34,35]. Megalin, in addition, mediates leptin signalling within the kidney via the Akt/mTOR signalling pathway to control cell metabolism, proliferation and consequently growth [36,37]. In our study, mTOR was significantly reduced in offspring from the HLA mothers. In a previous study, the loss of mTOR in nephron progenitor cells resulted in a failure to develop functional kidneys [38]. Again, surprisingly, the data in our study did not demonstrate a sex-specific effect on the Akt/mTOR signalling pathway.
Leptin, via the scavenger receptor megalin, also activates the Pi3k pathway [39]. In this study, megalin, leptin and Pi3kca were unaltered between treatment groups and between sexes. Similar results were observed in a uteroplacental insufficiency model of growth restriction, where megalin and its downstream signalling targets (mTOR, Pi3k, Ampka, Ampkb) at E20 were unaltered between restricted and control offspring [15]. Leptin and its downstream signalling mediators are critical in regulating cell proliferation, differentiation and growth. Therefore, the data from this study do not support the hypothesis that the leptin signalling pathway does not alter renal development in embryonic rats [15].
mTOR is a serine/threonine protein kinase within the Pi3k kinase family and serves as the subunit for two protein complexes, mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2) [40]. mTORC1 plays a key role in the production of proteins, lipids and nucleotides, and in suppressing autophagy, all of which are essential for cell growth [40]. Furthermore, mTOR inhibition suppresses general mRNA translation [41]. Thus, the reduced expression of mTOR shown in offspring of a maternal HLA model could have potentially greater effects on protein synthesis and mRNA translation in cell growth. Additionally under nutrient-sufficient conditions, mTORC1 phosphorylates ULK1, an autophagy-initiating kinase enzyme, preventing its activation by AMP-activated protein kinase (AMPK) [39,40]. Autophagy causes degradation of cell components in order to maintain cell activity and viability under nutrient-restricted conditions [39]. With both PRkab2 (AMPkb) and mTOR showing decreased expression in this current study, there is potential for reduced autophagy processes to occur which could possibly influence cell viability and therefore development, which warrants further investigation.
mTORC2 has a different role, controlling cell proliferation and survival via phosphorylation of several protein kinases [40]. One of the most important roles is the activation of Akt, a central actor in Pi3k signalling, via phosphorylation [40]. Upon activation of Akt, further phosphorylation and inhibition of key substrates occur to promote cell survival and proliferation [40]. Interestingly, in a mouse model, inhibition of mTOR with Rapamycin significantly decreased megalin, altering renal handling of proteins [42]. In this study, despite downregulation of mTOR, megalin mRNA was unaltered. Future studies should investigate the effects of the maternal HLA diet on megalin-mediated albumin endocytosis in offspring [42].
This current study showed that in fetal kidneys, leptin’s downstream signalling targets through ObR, including Jak2, Stat3 and Stat5a, were not altered in response to the maternal HLA diet. Leptin is produced in adipose tissue and the placenta [19]. In rats, the leptin surge occurs from around PN5 to PN15 [43], which is the time of completion of organogenesis. Therefore, the unaltered expression of leptin signalling targets at E20 should be reinvestigated at the time of the leptin surge. In previous investigations it was found that this postnatal leptin surge that occurs was reduced in offspring in cases of maternal undernutrition [44]. Maternal undernutrition, particularly protein restriction, was also found to reduce the expression of the downstream signalling target of leptin, Stat3 [45]. Unlike these findings, an exploration into the placenta associated with restricted mothers showed increased expression of Jak2, Stat3 and Stat5a mRNA [46]. Since we know leptin antagonism during the leptin surge results in reduced glomerular number and size and that offspring of restricted mothers (with resulting uteroplacental insufficiency) have a reduction in expression of leptin at PN7 [12], there is a clear indication that leptin plays a role in nephron formation at PN7 [19].
4. Materials and Methods
4.1. Experimental Animal Model and Diet
Ethical approval was granted by the Griffith University Animal Ethics Committee (NSC/01/17/AEC: 26 April 2017). Wistar Kyoto rats (8 weeks of age; n = 8 for diet with low linoleic acid (LLA) and n = 10 for HLA diet) were purchased from the Australian Resource Centre (Kensington, WA, Australia) and housed in accordance with the Australian Code of Practice for Care and Use of Animals for Scientific Purposes, following the ARRIVE Guidelines for Reporting Animal Research [47].
Eight-week-old female Wistar Kyoto (WKY) rats were housed in individually ventilated cages under a 12 h light–dark cycle at a temperature of 20–22 °C and provided with standard food pellets during acclimatisation and tap water ad libitum throughout the study. After a week for acclimatization, female rats were randomised to consume either a control low-LA (LLA: 1.44% of energy from LA, n = 8) or high-LA (HLA: 6.21% of energy from LA, n = 8) diet for 10 weeks. The minimum requirement for LA in the rodent diet is between 1 and 1.5% [48]. The composition of the custom diet has been previously reported [7]. These diets were isocaloric and matched for n-3 PUFA and total fat content. Pregnant females were euthanised at E20 via terminal anaesthetisation with intraperitoneal injections of sodium pentobarbital. The foetuses were euthanised by decapitation and fetal kidneys were harvested and stored at −80 °C following snap freezing in liquid nitrogen.
4.2. Quantitative Real-Time Polymerase Chain Reaction (qPCR)
Sex determination was performed as described previously [7]. Total RNA was extracted from kidney tissue using the RNeasy Mini kit (Qiagen, Chadstone, VIC, Australia) following the manufacturer’s guidelines. The quantification and evaluation of purity of RNA samples were conducted using a NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). Reverse transcription of RNA to synthesize complementary DNA was performed using the iScript gDNA clear cDNA synthesis kit (BioRad, Hercules, CA, USA) following the manufacturer’s guidelines. Quantitative PCR was performed using QuantiNova SYBR® green master mix (Qiagen) following manufacturer’s guidelines, in line with the Minimum Information for Publication of Quantitative Real-Time PCR Experiments (MIQE) guidelines [49]. PCR initial heat activation was run for 2 min at 95 °C, then qPCR reactions were run for 40 cycles of 95 °C for 5 s (denaturation) and 60 °C for 10 s (combined annealing/extension) using StepOneTM real-time PCR systems (Applied Biosystems, Waltham, MA, USA). Gene expression of targets (Supplementary Table S1) was quantified using the 2−ΔΔCq method normalised to the geometric mean of β-actin and β-2 microglobulin as reference genes. These reference genes were stable across the treatment groups.
4.3. Statistical Analysis
All data were analysed using GraphPad Prism 8.3.1. One male and one female offspring from each litter were analysed. n values represent individual offspring from separate litters. Data were analysed separately for males and females, with both sexes analysed by two-way ANOVA with maternal and postnatal diet as the factors. Specific comparisons were made using Tukey’s post hoc test. Data are presented as mean ± standard error of the mean (SEM). p-values < 0.05 were considered evidence of significant differences.
5. Conclusions
In conclusion, we have demonstrated that a maternal HLA diet in rodents alters genes responsible for branching morphogenesis and mTOR/AKT signalling. As these changes occurred independently of sex, this suggests that the reduction in maternal leptin and/or altered fatty acids significantly impacted embryonic nephrogenesis. Further studies should be conducted to identify if the maternal HLA diet alters adolescent or adult offspring renal function, in addition to offspring renal histological changes associated with a maternal HLA diet during pregnancy.
Acknowledgments
The authors would like to thank members of the laboratory and our collaborators for helpful discussions.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ijms25094688/s1.
Author Contributions
Conceptualization, A.J.M. and D.H.H.; methodology, J.S.M.C., O.J.H., A.V.P., A.J.M. and D.H.H.; formal analysis, N.S., O.J.H. and J.S.M.C.; resources, O.J.H., J.S.M.C., A.V.P., A.J.M. and D.H.H.; data curation, C.M., N.S., C.L.J. and A.E.B., writing—original draft preparation, C.M. and D.H.H.; writing—review and editing, all authors; supervision, O.J.H., J.S.M.C., A.V.P., A.J.M. and D.H.H.; project administration, D.H.H.; funding acquisition, A.J.M. and D.H.H. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Ethical approval was granted by the Griffith University Animal Ethics Committee (NSC/01/17/AEC: 26 April 2017).
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research was supported by the Allen Foundation, Inc. (DHH, AJM), and through the Australian Government’s Collaborative Research Networks (CRN) program (AJM). Scholarship funding is provided by Griffith University International Postgraduate Research Scholarship (GUIPRS-NS), Griffith University Postgraduate Research Scholarship (GUPRS-NS) and Griffith Health Top Up Scholarship (NS). This research was in part funded by the Allen Foundation (USA).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Naughton S.S., Mathai M.L., Hryciw D.H., McAinch A.J. Linoleic acid and the pathogenesis of obesity. Prostaglandins Other Lipid Mediat. 2016;125:90–99. doi: 10.1016/j.prostaglandins.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 2.Naughton S.S., Mathai M.L., Hryciw D.H., McAinch A.J. Australia’s nutrition transition 1961–2009: A focus on fats. Br. J. Nutr. 2015;114:337–346. doi: 10.1017/S0007114515001907. [DOI] [PubMed] [Google Scholar]
- 3.Ramsden C.E., Ringel A., Feldstein A.E., Taha A.Y., MacIntosh B.A., Hibbeln J.R., Majchrzak-Hong S.F., Faurot K.R., Rapoport S.I., Cheon Y., et al. Lowering dietary linoleic acid reduces bioactive oxidized linoleic acid metabolites in humans. Prostaglandins Leukot. Essent. Fat. Acids. 2012;87:135–141. doi: 10.1016/j.plefa.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simopoulos A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002;56:365–379. doi: 10.1016/s0753-3322(02)00253-6. [DOI] [PubMed] [Google Scholar]
- 5.Ailhaud G., Massiera F., Weill P., Legrand P., Alessandri J.M., Guesnet P. Temporal changes in dietary fats: Role of n-6 polyunsaturated fatty acids in excessive adipose tissue development and relationship to obesity. Prog. Lipid Res. 2006;45:203–236. doi: 10.1016/j.plipres.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Ortiz M., Álvarez D., Muñoz Y., Crisosto N., Valenzuela R., Maliqueo M. Linoleic and arachidonic fatty acids and their potential relationship with inflammation, pregnancy, and fetal development. Curr. Med. Chem. 2023 doi: 10.2174/0929867331666230706161144. in press . [DOI] [PubMed] [Google Scholar]
- 7.Shrestha N., Cuffe J.S.M., Holland O.J., Bulmer A.C., Hill M., Perkins A.V., Muhlhausler B.S., McAinch A.J., Hryciw D.H. Elevated maternal linoleic acid reduces circulating leptin concentrations, cholesterol levels and male fetal survival in a rat model. J. Physiol. 2019;597:3349–3361. doi: 10.1113/jp277583. [DOI] [PubMed] [Google Scholar]
- 8.Shrestha N., Holland O.J., Kent N.L., Perkins A.V., McAinch A.J., Cuffe J.S.M., Hryciw D.H. Maternal High Linoleic Acid Alters Placental Fatty Acid Composition. Nutrients. 2020;12:2183. doi: 10.3390/nu12082183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shrestha N., Sleep S., Helman T., Holland O., Cuffe J.S.M., Perkins A.V., McAinch A.J., Headrick J.P., Hryciw D.H. Maternal diet high in linoleic acid alters offspring fatty acids and cardiovascular function in a rat model. Br. J. Nutr. 2022;127:540–553. doi: 10.1017/s0007114521001276. [DOI] [PubMed] [Google Scholar]
- 10.Shrestha N., Sleep S.L., Holland O.J., Vidimce J., Bulmer A.C., Cuffe J.S.M., Perkins A.V., McAinch A.J., Hryciw D.H. Maternal Diet High in Linoleic Acid Alters Offspring Lipids and Hepatic Regulators of Lipid Metabolism in an Adolescent Rat Model. Int. J. Mol. Sci. 2024;25:1129. doi: 10.3390/ijms25021129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shrestha N., Vidimce J., Holland O.J., Cuffe J.S.M., Beck B.R., Perkins A.V., McAinch A.J., Hryciw D.H. Maternal and Postnatal High Linoleic Acid Diet Impacts Lipid Metabolism in Adult Rat Offspring in a Sex-Specific Manner. Int. J. Mol. Sci. 2021;22:2946. doi: 10.3390/ijms22062946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Attig L., Larcher T., Gertler A., Abdennebi-Najar L., Djaine J. Postnatal leptin is necessary for maturation of numerous organs in newborn rats. Organogenesis. 2011;7:88–94. doi: 10.4161/org.7.2.14871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Attig L., Solomon G., Ferezou J., Abdennebi-Najar L., Taouis M., Gertler A., Djiane J. Early postnatal leptin blockage leads to a long-term leptin resistance and susceptibility to diet-induced obesity in rats. Int. J. Obes. 2008;32:1153–1160. doi: 10.1038/ijo.2008.39. [DOI] [PubMed] [Google Scholar]
- 14.Moritz K.M., Dodic M., Wintour E.M. Kidney development and the fetal programming of adult disease. Bioessays. 2003;25:212–220. doi: 10.1002/bies.10240. [DOI] [PubMed] [Google Scholar]
- 15.Cuffe J.S.M., Briffa J.F., Rosser S., Siebel A.L., Romano T., Hryciw D.H., Wlodek M.E., Moritz K.M. Uteroplacental insufficiency in rats induces renal apoptosis and delays nephrogenesis completion. Acta Physiol. 2018;222:e12982. doi: 10.1111/apha.12982. [DOI] [PubMed] [Google Scholar]
- 16.Singh R.R., Cullen-McEwen L.A., Kett M.M., Boon W.M., Dowling J., Bertram J.F., Moritz K.M. Prenatal corticosterone exposure results in altered AT1/AT2, nephron deficit and hypertension in the rat offspring. J. Physiol. 2007;579:503–513. doi: 10.1113/jphysiol.2006.125773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gray S.P., Denton K.M., Cullen-McEwen L., Bertram J.F., Moritz K.M. Prenatal exposure to alcohol reduces nephron number and raises blood pressure in progeny. J. Am. Soc. Nephrol. 2010;21:1891–1902. doi: 10.1681/asn.2010040368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Short K.M., Smyth I.M. Branching morphogenesis as a driver of renal development. Anat. Rec. 2020;303:2578–2587. doi: 10.1002/ar.24486. [DOI] [PubMed] [Google Scholar]
- 19.Briffa J.F., McAinch A.J., Romano T., Wlodek M.E., Hryciw D.H. Leptin in pregnancy and development: A contributor to adulthood disease? Am. J. Physiol. Endocrinol. Metab. 2015;308:E335–E350. doi: 10.1152/ajpendo.00312.2014. [DOI] [PubMed] [Google Scholar]
- 20.Ahima R.S., Osei S.Y. Leptin signaling. Physiol. Behav. 2004;81:223–241. doi: 10.1016/j.physbeh.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 21.Briffa J.F., Grinfeld E., Poronnik P., McAinch A.J., Hryciw D.H. Uptake of leptin and albumin via separate pathways in proximal tubule cells. Int. J. Biochem. Cell Biol. 2016;79:194–198. doi: 10.1016/j.biocel.2016.08.031. [DOI] [PubMed] [Google Scholar]
- 22.van Abeelen A.F., de Rooij S.R., Osmond C., Painter R.C., Veenendaal M.V., Bossuyt P.M., Elias S.G., Grobbee D.E., van der Schouw Y.T., Barker D.J., et al. The sex-specific effects of famine on the association between placental size and later hypertension. Placenta. 2011;32:694–698. doi: 10.1016/j.placenta.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 23.Cheong J.N., Cuffe J.S., Jefferies A.J., Anevska K., Moritz K.M., Wlodek M.E. Sex-Specific Metabolic Outcomes in Offspring of Female Rats Born Small or Exposed to Stress During Pregnancy. Endocrinology. 2016;157:4104–4120. doi: 10.1210/en.2016-1335. [DOI] [PubMed] [Google Scholar]
- 24.Thomas K.N., Zimmel K.N., Roach A.N., Basel A., Mehta N.A., Bedi Y.S., Golding M.C. Maternal background alters the penetrance of growth phenotypes and sex-specific placental adaptation of offspring sired by alcohol-exposed males. FASEB J. 2021;35:e22035. doi: 10.1096/fj.202101131R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shrestha N., Melvin S.D., McKeating D.R., Holland O.J., Cuffe J.S.M., Perkins A.V., McAinch A.J., Hryciw D.H. Sex-Specific Differences in Lysine, 3-Hydroxybutyric Acid and Acetic Acid in Offspring Exposed to Maternal and Postnatal High Linoleic Acid Diet, Independent of Diet. Int. J. Mol. Sci. 2021;22:10223. doi: 10.3390/ijms221910223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pérez-Cerezales S., Ramos-Ibeas P., Rizos D., Lonergan P., Bermejo-Alvarez P., Gutiérrez-Adán A. Early sex-dependent differences in response to environmental stress. Reproduction. 2018;155:R39–R51. doi: 10.1530/rep-17-0466. [DOI] [PubMed] [Google Scholar]
- 27.Bazer F.W., Spencer T.E., Wu G., Cudd T.A., Meininger C.J. Maternal Nutrition and Fetal Development. J. Nutr. 2004;134:2169–2172. doi: 10.1093/jn/134.9.2169. [DOI] [PubMed] [Google Scholar]
- 28.Vega Q.C., Worby C.A., Lechner M.S., Dixon J.E., Dressler G.R. Glial cell line-derived neurotrophic factor activates the receptor tyrosine kinase RET and promotes kidney morphogenesis. Proc. Natl. Acad. Sci. USA. 1996;93:10657–10661. doi: 10.1073/pnas.93.20.10657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walker K.A., Bertram J.F. Kidney Development: Core Curriculum 2011. Am. J. Kidney Dis. 2011;57:948–958. doi: 10.1053/j.ajkd.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 30.Henry T., Abdallah M., Abdel-Hakeem A., Desai M., Nast C., Mansano R.Z., Toss M., Magee T. 458: Reduced nephrogenesis in low birth offspring is regulated by GDNF signaling dysregulation through the MAPK/ERK signaling pathway. Am. J. Obstet. Gynecol. 2008;199:S137. doi: 10.1016/j.ajog.2008.09.487. [DOI] [Google Scholar]
- 31.Abdel-Hakeem A.K., Henry T.Q., Magee T.R., Desai M., Ross M.G., Mansano R.Z., Torday J.S., Nast C.C. Mechanisms of impaired nephrogenesis with fetal growth restriction: Altered renal transcription and growth factor expression. Am. J. Obstet. Gynecol. 2008;199:252.e1–252.e7. doi: 10.1016/j.ajog.2008.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Costantini F. GDNF/Ret signaling and renal branching morphogenesis. Organogenesis. 2010;6:252–262. doi: 10.4161/org.6.4.12680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wood-Bradley R.J., Henry S.L., Barrand S., Giot A., Eipper L., Bertram J.F., Cullen-McEwen L.A., Armitage J.A. Analysis of structure and gene expression in developing kidneys of male and female rats exposed to low protein diets in utero. Anat. Rec. 2020;303:2657–2667. doi: 10.1002/ar.24417. [DOI] [PubMed] [Google Scholar]
- 34.Fisher C.E., Howie S.E.M. The role of megalin (LRP-2/Gp330) during development. Dev. Biol. 2006;296:279–297. doi: 10.1016/j.ydbio.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 35.Chai O.-H., Song C.-H., Park S.-K., Kim W., Cho E.-S. Molecular regulation of kidney development. Anat. Cell Biol. 2013;46:19–31. doi: 10.5115/acb.2013.46.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marwarha G., Dasari B., Prabhakara J.P.R., Schommer J., Ghribi O. β-Amyloid regulates leptin expression and tau phosphorylation through the mTORC1 signaling pathway. J. Neurochem. 2010;115:373–384. doi: 10.1111/j.1471-4159.2010.06929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fantus D., Rogers N.M., Grahammer F., Huber T.B., Thomson A.W. Roles of mTOR complexes in the kidney: Implications for renal disease and transplantation. Nat. Rev. Nephrol. 2016;12:587–609. doi: 10.1038/nrneph.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carney E. Regulation of nephrogenesis. Nat. Rev. Nephrol. 2018;14:536. doi: 10.1038/s41581-018-0033-3. [DOI] [PubMed] [Google Scholar]
- 39.Kim J., Kundu M., Viollet B., Kun-Liang G. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011;13:132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saxton R.A., Sabatini D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell. 2017;168:960–976. doi: 10.1016/j.cell.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thoreen C.C., Chantranupong L., Keys H.R., Wang T., Gray N.S., Sabatini D.M. A unifying model for mTORC1-mediated regulation of mRNA translation. Nature. 2012;485:109–113. doi: 10.1038/nature11083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gleixner E.M., Canaud G., Hermle T., Guida M.C., Kretz O., Helmstädter M., Huber T.B., Eimer S., Terzi F., Simons M. V-ATPase/mTOR signaling regulates megalin-mediated apical endocytosis. Cell Rep. 2014;8:10–19. doi: 10.1016/j.celrep.2014.05.035. [DOI] [PubMed] [Google Scholar]
- 43.Cottrell E.C., Cripps R.L., Duncan J.S., Barrett P., Mercer J.G., Herwig A., Ozanne S.E. Developmental changes in hypothalamic leptin receptor: Relationship with the postnatal leptin surge and energy balance neuropeptides in the postnatal rat. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2009;296:631–639. doi: 10.1152/ajpregu.90690.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Delahaye F., Breton C., Risold P.-Y., Enache M., Dutriez-Casteloot I., Laborie C., Lesage J., Vieau D. Maternal Perinatal Undernutrition Drastically Reduces Postnatal Leptin Surge and Affects the Development of Arcuate Nucleus Proopiomelanocortin Neurons in Neonatal Male Rat Pups. Endocrinology. 2008;149:470–475. doi: 10.1210/en.2007-1263. [DOI] [PubMed] [Google Scholar]
- 45.Rosario F.J., Jansson N., Kanai Y., Prasad P.D., Powell T.L., Jansson T. Maternal Protein Restriction in the Rat Inhibits Placental Insulin, mTOR, and STAT3 Signaling and Down-Regulates Placental Amino Acid Transporters. Endocrinology. 2011;152:1119–1129. doi: 10.1210/en.2010-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pérez-Pérez A., Maymó J., Dueñas J.L., Goberna R., Calvo J.C., Varone C., Sánchez-Margalet V. Leptin prevents apoptosis of trophoblastic cells by activation of MAPK pathway. Arch. Biochem. Biophys. 2008;477:390–395. doi: 10.1016/j.abb.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 47.Percie du Sert N., Hurst V., Ahluwalia A., Alam S., Avey M.T., Baker M., Browne W.J., Clark A., Cuthill I.C., Dirnagl U., et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. Br. J. Pharmacol. 2020;177:3617–3624. doi: 10.1111/bph.15193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choque B., Catheline D., Delplanque B., Guesnet P., Legrand P. Dietary linoleic acid requirements in the presence of α-linolenic acid are lower than the historical 2% of energy intake value, study in rats. Br. J. Nutr. 2015;113:1056–1068. doi: 10.1017/S0007114515000094. [DOI] [PubMed] [Google Scholar]
- 49.Bustin S.A., Benes V., Garson J.A., Hellemans J., Huggett J., Kubista M., Mueller R., Nolan T., Pfaffl M.W., Shipley G.L., et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009;55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are contained within the article and Supplementary Materials.