Abstract
Simple Summary
Lung cancer patients are frequently complicated by various respiratory diseases during their course. In particular, interstitial pneumonia and chronic obstructive pulmonary disease are often associated with lung cancer due to their common pathogenesis, and acute exacerbations of these diseases can be fatal. Therefore, it is important to select a therapy that is less likely to induce acute exacerbations of interstitial pneumonia and chronic obstructive pulmonary disease. Furthermore, lung cancer patients are at high risk of developing or reactivating pulmonary tuberculosis triggered by pharmacotherapy and often struggle with the diagnosis and treatment of tuberculosis complicated by lung cancer. This review summarizes the current evidence regarding pharmacotherapy for lung cancer patients with interstitial pneumonia, chronic obstructive pulmonary disease, and pulmonary tuberculosis and discusses future prospects.
Abstract
Non-small cell lung cancer (NSCLC) patients are often complicated by other respiratory diseases, including interstitial pneumonia (IP), chronic obstructive pulmonary disease (COPD), and pulmonary tuberculosis (TB), and the management of which can be problematic. NSCLC patients with IP sometimes develop fatal acute exacerbation induced by pharmacotherapy, and the establishment of a safe treatment strategy is desirable. For advanced NSCLC with IP, carboplatin plus nanoparticle albumin-bound paclitaxel is a relatively safe and effective first-line treatment option. Although the safety of immune checkpoint inhibitors (ICIs) for these populations remains controversial, ICIs have the potential to provide long-term survival. The severity of COPD is an important prognostic factor in NSCLC patients. Although COPD complications do not necessarily limit treatment options, it is important to select drugs with fewer side effects on the heart and blood vessels as well as the lungs. Active TB is complicated by 2–5% of NSCLC cases during their disease course. Since pharmacotherapy, especially ICIs, reportedly induces the development of TB, the possibility of developing TB should always be kept in mind during NSCLC treatment. To date, there is no coherent review article on NSCLC with these pulmonary complications. This review article summarizes the current evidence and discusses future prospects for treatment strategies for NSCLC patients complicated with IP, severe COPD, and TB.
Keywords: acute exacerbation, chronic obstructive pulmonary disease, cytotoxic anti-cancer drug, immune checkpoint inhibitor, interstitial pneumonia, lung cancer, non-small cell lung carcinoma, tuberculosis
1. Interstitial Pneumonia
1.1. Introduction
Approximately 5–15% of lung cancer (LC) patients have interstitial pneumonia (IP) at the time of diagnosis [1,2,3]. Contrarily, the incidence of LC in patients with IP is reported to be 10–20%, which is 5–14 times higher than the incidence in the general population [1,4]. The common risk factors for both IP and LC include smoking, bacterial and viral infections, environmental and occupational exposures, and chronic tissue damage [5,6]. Additionally, the common molecular mechanisms include genetic and epigenetic mutations, abnormal micro-RNA expression, inhibition of apoptosis associated with the activation of intracellular signal transduction, and weakening of cellular interactions [7,8]. These common etiologies result in a high rate of LC and IP complications.
Several studies have reported that LC patients with IP have a worse prognosis than patients with IP alone or LC alone. In the Hokkaido study, a large observational study involving 553 idiopathic pulmonary fibrosis (IPF) patients in Japan, LC was the third leading cause of death (11%) [9]. In an observational study involving 181 IPF patients, Tomassetti et al. reported that the median survival of IPF patients with LC was significantly shorter than that of IPF patients without LC (38.7 vs. 63.9 months, p < 0.001) [10]. Additionally, in a retrospective study of 637 LC patients to determine the impact of IPF on prognosis, 34 (5.3%) LC patients with IPF had a worse prognosis than those without IPF, regardless of the stage or treatment received [11].
Despite the high complication rate and poor prognosis of LC patients with IP, almost all clinical trials for LC exclude this population because of concerns about chemotherapy-induced acute exacerbations of IP. Acute exacerbation is a fatal complication in IP patients, with a mortality rate of 30–50% [12,13]. Patients with IPF develop acute exacerbations at a frequency of 10–15% per year during the natural course of the disease, and patients with IP other than IPF, including non-specific interstitial pneumonia and IP associated with collagen vascular disease, also develop acute exacerbations at a frequency of 3–5% per year [14,15,16]. Furthermore, the incidence of chemotherapy-induced acute exacerbations of IP has been reported to be 5–20%; thus, it is most important to select a treatment that is less likely to induce acute exacerbations of pre-existing IP [17,18,19].
Recently, LC treatment has made significant advances, and newer therapies such as carbon-ion radiotherapy and high-power microwaves are expected to be relatively safe treatment options for LC patients with IP complications [20,21]. However, most LC patients are already advanced at diagnosis, and pharmacotherapy remains the cornerstone of treatment. In particular, clinicians are often faced with the difficult choice of pharmacotherapy for patients with non-small cell lung carcinoma (NSCLC), which has various treatment options, including molecular-targeted drugs and immune checkpoint inhibitors (ICIs), in addition to cytotoxic chemotherapy. In the following sections, we summarize the current evidence and discuss future prospects for pharmacotherapy for advanced NSCLC with comorbid IP.
1.2. Risk of Acute Exacerbation by Pharmacotherapy for NSCLC with Comorbid IP
Based on previous reports, pharmacotherapy for NSCLC patients with comorbid IP induces acute exacerbation of IP with a frequency of approximately 5–20% [17,18,19]. The exact mechanism by which pharmacotherapy causes acute exacerbations of IP is not known, but direct cell damage by reactive oxygen species and proteolytic enzymes and activation of immune cells have been proposed [22]. The risk factors for the development of acute exacerbations of IP due to cytotoxic chemotherapy have been reported in several studies. The patients with the usual interstitial pneumonia (UIP) pattern on computed tomography (CT) reportedly have a higher frequency of acute exacerbations induced by cytotoxic chemotherapy than those with a non-UIP pattern (30% vs. 8%, p = 0.005) [23]. Additionally, low forced vital capacity (FVC) is also reported to be associated with a higher risk of acute exacerbation induced by cytotoxic chemotherapy, and low FVC is suggested to be more strongly associated with the risk of acute exacerbation than UIP pattern on CT [24]. Additionally, a modified GAP index, a scoring system to evaluate IPF severity, is also reported to be associated with a higher incidence of chemotherapy-induced acute exacerbations and a lower 1-year survival rate [25,26]. Although there are no established risk factors or biomarkers that can actually predict chemotherapy-induced acute exacerbation, these reports suggest that the administration of chemotherapy for NSCLC patients with IP should be considered based on a comprehensive assessment of the patient’s age, general condition, IP severity, and LC prognosis, with careful consideration of the risk–benefit ratio.
In the pharmacotherapy for NSCLC with comorbid IP, it is important to identify and select drugs that are unlikely to cause acute exacerbations of IP. The Japanese Respiratory Society issued a “Statement” on the treatment of LC with comorbid IP in 2017 [27]. This statement classified cytotoxic chemotherapy into three categories according to the risk of acute exacerbation of IP, and several chemotherapy agents, including platinum-containing drugs, etoposide, paclitaxel, and vinorelbine, are classified as drugs that can be administered with caution. In actual practice, first-line treatment may be considered from among these treatment options reported to be relatively safe. However, there is no evidence of efficacy beyond the second-line treatment, and it is not recommended at this time.
ICIs are often reported to carry a high risk of causing acute exacerbation of pre-existing IP [28,29]. A Japanese retrospective study of NSCLC patients treated with ICIs showed a higher incidence of pneumonitis in patients with IP than in those without IP (29% vs. 10%, p = 0.027) [30]. Furthermore, a recent meta-analysis of chemotherapy, including ICIs for LC with comorbid IP, showed that ICI-associated pneumonitis occurred at a high rate of 27–30% for all grades and 12–15% for grade 3 or higher [31,32]. Based on these results, ICIs are generally considered to be at a high risk for acute exacerbation of IP. However, in these reports, whether there are differences in the risk for developing acute exacerbations due to the pre-existing IP subtypes, the specific radiological findings, or pulmonary function has not been fully investigated. In pilot (N = 6) and phase II (N = 18) trials of nivolumab in previously treated NSCLC patients with mild IP defined as (1) no honeycomb lung, (2) negative autoantibody, and (3) %VC ≧ 80% (Commonly called “HAV Criteria”, an acronym for Honeycomb lung, autoantibody, and VC), the frequency of drug-induced pneumonitis ranged from 0–11% [33,34]. On the other hand, in a phase II trial of atezolizumab in NSCLC patients with chronic fibrotic IP, with %FVC > 70%, with or without honeycomb lung, grade 3 or higher pneumonitis occurred at a rate of 24% [35]. The post-hoc analysis of this study suggested that the presence of honeycomb lung may be a risk factor for pneumonitis, although it is not statistically significant. Meanwhile, the aforementioned meta-analysis of ICIs for NSCLC patients with IP, which included three interventional studies mentioned above and seven retrospective studies, reported that honeycomb lung may not be a risk factor for pneumonitis [33]. Based on these results, the safety of ICIs for NSCLC patients with IP has not yet been established, and currently, there is no rationale for its use in first-line therapy. The risk factors for acute exacerbations of IP caused by ICIs are still unknown, and further accumulation of a large number of data and a detailed analysis of the risk factors are required in the future.
For NSCLC with driver gene mutations/translocations, including epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase, and c-ros oncogene 1 (ROS1) genes, the first-line therapy with tyrosine kinase inhibitors (TKIs) targeting gene mutation/translocation is recommended. However, when gefitinib-induced pneumonitis occurred frequently in Japan, pre-existing IP was identified as an independent risk factor for pneumonitis [17,36]. Since then, special caution has been required when administering molecular-targeted drugs to driver gene mutation-positive NSCLC patients with comorbid IP. In fact, it has been reported that only 0.4% of lung adenocarcinoma patients with EGFR mutations have IP; thus, there are few situations in real-world clinical practice where we wonder whether EGFR-TKIs should be administered to these populations [37]. Contrarily, with regard to gene mutations, such as KRAS and BRAF, which are relatively common among smokers, patients may potentially have a higher frequency of IP complications. In fact, it has been suggested that NSCLC patients with IP may have more KRAS and BRAF mutations than those without IP [38]. A previous case study reported the KRAS inhibitor sotrasib being administered safely and effectively to KRAS G12C positive NSCLC patients with comorbid IP [39]. There are no detailed data on the prevalence of IP or risk factors for acute exacerbation of IP in NSCLC patients with driver mutations/translocations other than EGFR, and further studies are still needed.
1.3. First-Line Treatment of NSCLC with Comorbid IP
Table 1 summarizes the prospective clinical trials reported to date on the first-line treatment of advanced NSCLC with comorbid IP.
Table 1.
Major prospective study on non-small cell lung carcinoma complicated by interstitial pneumonia.
| Line | Phase | Study Design | Treatment Regimen | Number | PFS | OS | ORR | Incidence of AE of IP | Reference |
|---|---|---|---|---|---|---|---|---|---|
| First-line | 2 | Single arm | CBDCA + nab-PTX | 94 | 6.2 | 15.4 | 51% | 4.3% | [40] |
| First-line | 2 | Single arm | CBDCA + nab-PTX | 36 | 5.3 | 25.4 | 55.6% | 5.6% | [41] |
| First-line | Pilot | Single arm | CBDCA + weekly PTX | 18 | 10.6 | 61% | 5.6% | [18] | |
| First-line | 2 | Single arm | CBDCA + weekly PTX | 35 | 6.3 | 19.8 | 69.7% | 12.1% | [42] |
| First-line | Pilot | Single arm | CBDCA + S-1 | 21 | 4.2 | 9.7 | 33.0% | 9.5% | [43] |
| First-line | 2 | Single arm | CBDCA + S-1 | 33 | 4.8 | 12.8 | 33.3% | 6.1% | [44] |
| First-line | 2 | Single arm | CBDCA + weekly PTX + Bev | 17 | 5.7 | 12.9 | 52.9% | 5.9% | [45] |
| First-line | 3 | Randomized control trial | CBDCA + nab-PTX | 120 | 5.5 | 13.0 | 56.0% | 1.6% | [46] |
| CBDCA + nab-PTX + Nintedanib | 120 | 6.2 | 15.3 | 69.0% | 4.1% | ||||
| Second-line | Pilot | Single arm | Nivolumab | 6 | 50% | 0.0% | [33] | ||
| Second-line | 2 | Single arm | Nivolumab | 18 | 7.4 | 15.6 | 39% | 11.1% | [34] |
| Second-line | 2 | Single arm | Atezolizumab | 17 (Stopped) |
3.4 | 6.3% | 29.4% | [35] |
Abbreviations: PFS, progression-free survival; OS, overall survival; ORR, overall response rate; AE, acute exacerbation; IP, interstitial pneumonia; CBDCA, carboplatin; nab-PTX, nanoparticle albumin-bound paclitaxel; PTX, paclitaxel; Bev, bevacizumab.
First, carboplatin plus nanoparticle albumin-bound paclitaxel (nab-paclitaxel) may be considered as first-line chemotherapy for advanced NSCLC with comorbid IP. Until now, two single-arm phase II trials of carboplatin plus nab-paclitaxel in advanced NSCLC with comorbid IP, which were multicenter prospective studies involving a relatively large number of patients (94 and 36 patients, respectively), have been reported [40,41]. Both studies showed consistent safety and efficacy results with an incidence of acute exacerbation of existing IP of 4.3–5.6%, response rates of 51–56%, median progression-free survival (PFS) of 5.3–6.2 months, and median overall survival (OS) of 15.1–15.4 months. Based on these results, this regimen may be considered as standard first-line treatment for NSCLC comorbid with IP.
Two prospective trials also investigated the combination therapy of carboplatin plus weekly paclitaxel for advanced NSCLC with comorbid IP [18,42]. Although these trials involved fewer patients than the aforementioned trials (35 and 18 patients, respectively), the incidence of acute exacerbation of IP induced by this combination therapy has been reported at 5.6–12.1%, the response rate of 61–70%, median PFS of 5.3–6.3 months, and median OS of 10.6–19.8 months suggesting that this may also be a promising treatment option.
Additionally, two prospective trials of carboplatin plus S-1 in 21 and 33 patients, respectively, reported acute exacerbation rates of 6.1–9.5%, response rates of 33.0–33.3%, median PFS of 4.2–4.8 months, and median OS of 9.7–12.8 months [43,44]. Based on these results, carboplatin plus S-1 therapy may be another option for the first-line treatment for NSCLC comorbid with IP. However, instead of using S-1 in the first-line treatment, preservation of S-1 for the second-line treatment, described below, may also be considered.
The addition of angiogenesis inhibitors, including bevacizumab or ramucirumab, for NSCLC patients comorbid with IP, is controversial but may be relatively safe. In a retrospective study of 51 NSCLC patients with IP, those treated with bevacizumab had better rates of acute exacerbation of IP (0% vs. 22.6%) and median PFS (8.0 vs. 4.3 months) than those treated without bevacizumab [47]. Similarly, several retrospective studies have shown that the combination of bevacizumab was relatively safe to use in NSCLC patients with IP [48,49]. Recently, a multicenter prospective phase II study of carboplatin plus weekly paclitaxel plus bevacizumab for advanced NSCLC with comorbid IP was conducted in Japan [45]. Although this was a single-arm study of 17 patients, this study showed relatively safe and efficacy results with an incidence of acute exacerbation of existing IP of 5.9%, response rates of 52.9%, median PFS of 5.7 months, and median OS of 12.9 months. From these results, the concomitant use of bevacizumab is unlikely to change the risk of acute exacerbations of IP and may be considered in patients who are eligible for administration.
Recently, monotherapy with ICIs or combination therapy with ICIs and cytotoxic chemotherapy has become the standard of care for the first-line treatment of advanced NSCLC. However, as noted above, many studies have shown that pre-existing IP increases the risk of ICI-associated pneumonitis, and most ICI package inserts state that they should be administered with caution to LC patients with comorbid IP [30,31,32]. Therefore, ICIs should not be used as first-line treatment for NSCLC patients with IP at this time.
1.4. Second-Line Treatment of NSCLC with Comorbid IP
To date, no prospective interventional study of cytotoxic chemotherapy as second or subsequent-line therapy for NSCLC with comorbid IP has been reported. In a nationwide survey on second-line therapy of 278 LC patients with IP in Japan, the acute exacerbation rate of IP was reported for each cytotoxic anti-cancer drug, with a frequency of 15.3% for docetaxel and 28.6% for pemetrexed [50]. Since other retrospective studies have also reported a relatively high rate of acute exacerbations of IP with these agents, docetaxel and pemetrexed should be recognized as relatively high-risk drugs for NSCLC patients with comorbid IP [51,52,53]. S-1 monotherapy is thought to be relatively less likely to induce acute exacerbations of IP based on the results of retrospective studies. According to the aforementioned national survey for LC patients with comorbid IP, no patient experienced an acute exacerbation of pre-existing IP when treated with S-1 alone [50]. Additionally, as mentioned above, the low incidence of acute exacerbations (6.1–9.5%) was reported in the two prospective studies of carboplatin plus S-1 as first-line treatment [43,44]. On the other hand, another retrospective study reported that even S-1 caused acute exacerbation of IP in 21% of cases (3 of 14 patients) [23]. From these results, S-1 for NSCLC with comorbid IP may be less risky than second-line therapy with other cytotoxic agents, but caution should, of course, be exercised when administered.
The safety of ICIs for NSCLC patients with IP has not yet been established, but it has been suggested that ICIs may be highly effective for this population. In a pilot study of 6 NSCLC patients with mild IP that met the so-called “HAV criteria” (without honeycomb lung, without autoantibody, and %VC ≥ 80%), nivolumab monotherapy did not induce acute exacerbation of IP [33]. Furthermore, in a phase II study of 18 NSCLC patients with mild IP selected from four centers using the same criteria, nivolumab-induced pneumonitis in two patients (11%), all of whom were grade 2 and improved rapidly with corticosteroid therapy [34]. Importantly, these trials reported a high efficacy of nivolumab monotherapy in NSCLC patients with IP, with response rates of 39–50% and disease control rates of 72–100%. Similarly, a multicenter, retrospective study of 216 patients with NSCLC reported that IP patients tended to have a higher response rate than those without IP (27% versus 13%, p = 0.078) [54]. Because the development of IP is often associated with smoking and microsatellite instability, it has been suggested that NSCLC with comorbid IP with a high tumor mutation burden may benefit more from ICIs [7]. On the other hand, however, a phase II trial of atezolizumab in NSCLC patients with IP terminated early after the enrollment of 17 patients due to a high incidence of pneumonitis with 24% for grade 3 or higher, and 6% for grade 5 [35]. In this study, logistic regression analysis suggested that the presence of honeycomb lungs may be associated with the development of ICI-induced pneumonitis, although the results were not statistically significant.
Based on these results, S-1 monotherapy is often administered as standard second-line treatment of NSCLC with comorbid IP. However, retrospective studies of cytotoxic chemotherapy as a second-line treatment for NSCLC with IP have shown limited efficacy with 1-year survival rates of approximately 10% and little promise for long-term survival [52,53]. Therefore, for NSCLC patients with IP, who have few treatment options and a poor prognosis, ICIs have the potential to be the only existing therapy with long-term survival. Large studies identifying the risk factors for ICI-induced pneumonitis are needed for appropriate patient selection.
1.5. Antifibrotic Agents for NSCLC with Comorbid IP
Recently, antifibrotic agents such as pirfenidone and nintedanib have been widely used clinically worldwide for the treatment of IPF. Antifibrotic agents have been reported to slow the progression of IP and reduce the incidence of acute exacerbations of IP. In a randomized phase III trial in IPF, nintedanib significantly prevented acute exacerbations of IP during the course of the disease (1.9% and 4.7%, p = 0.010), with a hazard ratio of 0.53 for the time to the first acute exacerbation [55]. Furthermore, in a multicenter phase II trial of perioperative treatment of NSCLC with IPF, pirfenidone reduced the incidence of postoperative acute exacerbations of IPF [56].
Based on these results, the J-SONIC trial, a randomized phase III trial of carboplatin plus nab-paclitaxel with or without nintedanib for NSCLC patients with IPF, was conducted in Japan [46]. However, this study failed to demonstrate a reduction in the incidence of acute exacerbations of IP with concomitant nintedanib, and at this time, there is no evidence to aggressively recommend concomitant antifibrotic therapy for advanced NSCLC patients with comorbid IP.
2. Chronic Obstructive Pulmonary Disease
2.1. Introduction
Chronic obstructive pulmonary disease (COPD) and LC, both of which are mainly caused by smoking, are often combined, and the prevalence of COPD in LC patients is approximately 40–70% [57,58]. The impact of COPD on the survival of LC patients varies from report to report, but a large meta-analysis of the United States (U.S.), European, and Asian studies reported that LC patients with COPD have a poorer prognosis than those without COPD (HR, 1.17; 95% CI: 1.10–1.25) [59]. Other reports have also generally recognized COPD as a poor prognostic factor for LC patients [60,61,62]. Furthermore, the severity of COPD is considered an important prognostic factor in LC patients, and it has been reported that the more severe the Global Initiative for Chronic Obstructive Lung Disease classification grade of COPD severity, the worse the prognosis of LC patients [63]. No molecular pathway linking COPD severity and LC prognosis has been identified, and elucidation of this molecular pathway may contribute to treatment selection for LC patients with severe COPD. LC patients with severe COPD have not only low pulmonary function but also a high frequency of systemic complications, including cancer cachexia, heart failure, and diabetes mellitus, and many patients have poor performance status (PS). Thus, the decision to use pharmacotherapy for LC patients with severe COPD should be carefully considered.
In several large retrospective studies, the presence of mild-to-moderate COPD with a preserved percent-predicted forced expiratory volume in one second (%FEV1) of >50% did not significantly affect the prognosis of LC or the side effects of treatment [58,64]. Patients with severe COPD requiring home oxygen therapy have been excluded from many clinical trials and studies, and there are few reports on chemotherapy for LC. However, a retrospective observational study has reported that even advanced NSCLC patients with severe to most severe COPD had a longer OS with chemotherapy than with supportive care alone (14.0 vs. 8.0 months, p = 0.003) [65]. The Japanese retrospective study evaluating the efficacy and safety of chemotherapy in 40 patients with advanced LC complicated by chronic respiratory failure requiring home oxygen therapy also showed that the only factor significantly associated with improved prognosis was the use of first-line or second-line treatment (HR, 0.42; 95% CI: 0.18–0.94) [66]. Therefore, even in the cases of low pulmonary function requiring home oxygen therapy, up to the first-line or second-line treatment may be considered if the PS is maintained.
In the following sections, we summarize the current evidence and discuss future issues regarding pharmacotherapy for advanced NSCLC with severe COPD.
2.2. Cytotoxic Chemotherapy for NSCLC with Severe COPD
There is no clear evidence for the efficacy and safety of cytotoxic chemotherapy for severe COPD requiring home oxygenation, and no drugs are listed as contraindicated for severe COPD in the Japanese drug package insert. However, these patients with low pulmonary function can be fatal even with mild infectious pneumonia and drug-induced pneumonitis. LC patients with severe or most severe COPD (%FEV1 < 50%) reported a significantly higher rate of pulmonary adverse events such as pneumonia (46.4% vs. 31.2%, p < 0.001) and COPD exacerbations (30.4% vs. 6.9%, p < 0.001) during LC treatment than those with mild-to-moderate COPD (%FEV1 > 50%) [67]. Therefore, it is important to select cytotoxic chemotherapy with a relatively low risk of pulmonary adverse events in LC patients with severe COPD.
Additionally, COPD patients are often complicated by other comorbidities, including cardiovascular disease and diabetes, which may affect patient survival [68]. Especially in patients with severe COPD, cytotoxic chemotherapy that affects the cardiovascular system should be avoided because of the risk of worsening respiratory status even with increased cardiac load due to hypertension or massive infusion. In light of these points, carboplatin should be chosen for platinum doublet therapy because cisplatin may aggravate heart failure due to the massive infusions. Similarly, docetaxel, often used in the chemotherapy of NSCLC, carries the risk of increased cardiac load due to fluid retention and edema. Furthermore, a retrospective study involving 392 NSCLC patients reported that LC patients with emphysema are 4.95 times more likely to develop pneumonitis induced by docetaxel than patients without emphysema [69]. For these reasons, the use of docetaxel in NSCLC patients with severe COPD should be cautious.
COPD affects the expression of vascular endothelial growth factor (VEGF) in the circulation, suggesting that the anti-VEGF and anti-VEGF receptor inhibitors may be effective in NSCLC patients with COPD. A small retrospective study of NSCLC patients treated with carboplatin plus paclitaxel plus bevacizumab reported significantly longer PFS in patients with COPD (35 patients) than in those without COPD (39 patients) (6.1 vs. 3.3 months, p = 0.049) [70]. Thus, anti-VEGF and anti-VEGF receptor inhibitors should be considered for NSCLC patients with mild-to-moderate COPD. However, given the risk of increased cardiac burden due to fluid retention and edema, which are typical adverse events of anti-VEGF and anti-VEGF receptor inhibitors, caution should be exercised when administering these drugs to NSCLC patients with severe to most severe COPD.
In view of the above, in clinical practice, cytotoxic chemotherapy, such as pemetrexed, paclitaxel, nab-PTX, and S1 as single agents or in combination with carboplatin, is often empirically selected for NSCLC with severe COPD. To date, however, there have been no prospective trials of cytotoxic chemotherapy for this population, and the optimal treatment of choice is unknown. To resolve these clinical questions, it is necessary to accumulate data from large-scale studies in the future.
2.3. Immune Checkpoint Inhibitors for NSCLC with Severe COPD
Recently, it has been suggested that in patients with NSCLC, COPD comorbidity may increase the efficacy of ICIs [71,72]. A retrospective cohort study of 125 NSCLC patients treated with ICIs reported that COPD patients and current smokers had longer PFS [73]. Additionally, a subset analysis of this study reported significantly longer survival in ex-smokers with COPD complications than in ex-smokers without COPD (OS; 359 vs. 145 days, p = 0.035). COPD patients reportedly have altered Th1/Th2 ratios and Treg/Th17 balance due to increased Th1 and Th2 cells and increased expression of PD-1 on CD8+ T cells and PD-L1 on macrophages, which may increase the efficacy of ICIs [73,74].
On the other hand, it has been reported that a complication of COPD increases the risk of developing ICI-induced pneumonitis. The U.S. Food and Drug Administration (FDA) report on pembrolizumab, an anti-PD-1 antibody drug, found that patients with COPD had a higher incidence of pneumonitis than those without COPD (5.4% vs. 3.1%) [75]. Similarly, a recent multicenter retrospective study of ICI-related pneumonitis from the U.S. also reported that the presence of COPD increased the risk of developing pneumonitis by 2.79 times [76]. Furthermore, in a retrospective study involving 99 NSCLC patients with COPD treated with ICIs, a higher incidence of immune-related adverse events and poorer prognosis were observed in patients with more severe COPD [77]. Thus, ICI administration should be cautious in patients with severe COPD, who are at a high risk of developing ICI-related pneumonitis, which can be fatal due to the low respiratory reserve.
Additionally, COPD patients with a history of heavy smoking are often complicated by IP, and they are so-called combined pulmonary fibrosis and emphysema. As mentioned above, IP is another risk factor for the development of ICI-related pneumonitis. The stronger the emphysematous changes, the more difficult it is to determine whether IP is complicating the disease. If it is difficult to determine the presence or absence of IP complications, fine crackles on auscultation may help in making a decision [78].
2.4. Treatment of COPD for NSCLC with COPD
When treating LC patients with COPD, clinicians often focus solely on LC treatment and neglect the treatment of the COPD. Although there is no clear indication yet that the medical management of COPD is necessary for LC patients, many studies have shown that COPD affects the prognosis and response to chemotherapy of LC. In a small Japanese retrospective study, among the NSCLC patients with COPD, those treated for COPD with a long-acting muscarinic antagonist and/or long-acting β2 agonist (N = 37) had significantly longer survival than those not treated (N = 66) (16.7 vs. 8.2 months, p = 0.023) [79]. Surprisingly, approximately two out of three NSCLC patients with COPD in this study were not receiving treatment for COPD. This study also reported a positive impact of COPD treatment on prognosis in a multivariate analysis (HR, 0.52; 95% CI: 0.31–0.87), suggesting that it may be important to treat COPD as well as LC.
3. Pulmonary Tuberculosis
3.1. Introduction
Patients with tuberculosis (TB) have been reported to have a higher risk of developing LC than healthy individuals. A population-based cohort study in Taiwan showed that a history of TB increased the risk of developing LC by 1.76 times [80]. A recent large-scale prospective cohort study analyzing national medical examination data in Korea also found that the presence of TB increases the incidence of LC (HR: 1.49 for men and 1.37 for women) [81]. Conversely, patients with malignant diseases have also reported a high risk of developing active TB during their course. A large meta-analysis in the U.S. reported a particularly high risk of developing TB in hematologic cancers, head and neck cancers, and LC (incidence rate ratios = 26, 16, and 9, respectively) [82]. An observational study of 904 LC patients in Japan reported a cumulative incidence of TB of 1.38%, which is 25 times higher than that of the general population [83]. To summarize the previous Japanese reports on LC and TB, active TB is noted in 2–5% of LC cases, whereas LC is noted in 1–2% of active TB cases [84,85,86,87,88].
The mechanism of TB complicated with LC has been postulated to include proliferative changes in the columnar epithelium of the bronchial wall and squamous cell transformation due to chronic inflammation and oxidative stress of TB, as well as immune evasion of tumor cells due to the changes in cellular immune function caused by TB infection [89,90,91].
When a patient with LC is complicated with TB, there are several clinical problems. First, when a new shadow due to TB appears during the disease course of an LC patient, diagnosing TB is difficult because of the wide variety of differential diagnoses. Second, when treating TB in patients with LC, it is difficult to determine whether treatment for LC should be continued or discontinued. Additionally, the interactions between drugs for TB and LC need to be considered. In the following section, we summarize the evidence to date on how to manage when LC patients develop TB.
3.2. Diagnosis of TB Complicated by LC Patients
As noted above, LC patients with reduced cellular immunity are at a high risk of developing active TB. In addition to known risk factors, including older age, male sex, history of TB, and gastrectomy, treatment for LC, including cytotoxic chemotherapy and ICIs, increases the risk of developing TB [92,93,94]. Notably, a series of cases of TB developing during the course of ICI treatment have recently been reported [95,96,97,98]. In a Japanese retrospective study involving 297 LC patients treated with ICIs, five patients (1.7%) developed TB during the treatment course [99]. Importantly, three out of the five patients had pulmonary TB, whereas the other two had extrapulmonary TB (cervical and hilar lymph nodes and knee joints), suggesting that TB development during the ICI treatment requires attention not only to the lungs but also to the whole body. Interestingly, in a study analyzing the FDA database, the use of anti-PD-1 or PD-L1 antibodies was associated with the development of TB, whereas the use of anti-CTLA-4 antibodies did not increase the risk of developing TB [100]. The etiology of TB induced by ICI treatment is speculated to be a mechanism by which Mycobacterium tuberculosis evades the host immune response by inhibiting PD-1, which induces suppression of IFN-γ production and over-secretion of TFN-α [90,98]. Therefore, when treating LC patients with pharmacotherapy, especially anti-PD-1/PD-L1 antibodies, the risk of developing TB should always be kept in mind.
When LC and TB are combined, the diagnosis of either disease is often delayed because of the difficulty in differentiating the two diseases from each other, and this delay worsens the patient’s prognosis [101,102,103]. Therefore, it is important to detect and diagnose TB in LC patients as early as possible. Despite the good response to systemic chemotherapy in LC patients, the emergence of isolated sites or new foci, especially in typical anatomical sites of TB infection (e.g., upper lobes or apical lower lobes of the lung), should draw attention to the possibility of complicating a TB infection [104]. A previous retrospective study comparing LC patients with and without TB found that LC patients with TB were more likely to have symptoms such as cough, hemoptysis, nocturnal sweating, and more diverse CT findings such as lobar signs of mass, calcified lesions, pleural thickening, and hemorrhagic pleural effusion [105,106]. If these findings are present in LC patients, the possibility of developing TB complications should be considered.
Several meta-analyses and position statements have recommended that the IFN-γ releasing assay (IGRA) be performed as a screening procedure prior to the initiation of systemic chemotherapy, especially ICIs [82,107,108]. On the other hand, patients with malignant tumors were more likely to have false-negative results and weaker responses to IGRA due to their decreased immunocompetence [109,110,111]. Thus, although the usefulness of IGRA as a screening tool prior to chemotherapy for LC is still controversial, the IGRA measurements may be considered prior to ICIs in LC patients with lung lesions suggestive of chronic infection or at risk for the aforementioned TB infection [96].
3.3. Treatment Strategy for LC Patients with TB
Reports on the efficacy and safety of therapy for LC during TB treatment are limited, and there is no clear evidence yet. However, several reports have shown that cancer treatment does not interfere with TB treatment. A retrospective study conducted in Japan involving 30 patients with malignant tumors complicated by TB (including 15 LC patients) reported that concurrent administration of anti-TB drugs and cytotoxic chemotherapy was effective and safe [103]. Similarly, in a Korean retrospective case–control study, TB that developed during chemotherapy was not clinically different from TB that developed under normal circumstances, and chemotherapy did not interfere with TB treatment [92]. Recently, it has been reported that the negative conversion rate of sputum culture with standard TB treatment after 2 months was good at 94%, even in LC patients with TB; furthermore, no significant difference was observed in the outcome of LC treatment between the LC patients with and without TB complications [94,112].
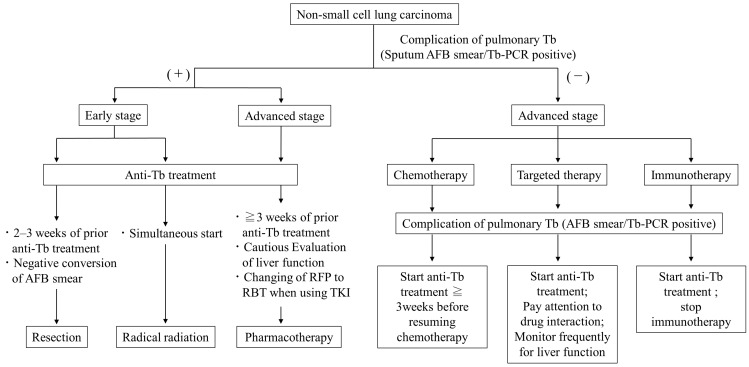
There are no clear criteria for when to discontinue or resume lung cancer treatment in LC patients with active TB. The risk of infecting medical staff with TB must also be considered, and ideally, it may be desirable to precede TB treatment if LC treatment, such as chemotherapy, can be postponed. Because the duration of isolation hospitalization for TB is approximately 2 months, it is sometimes possible to treat LC without missing the treatment opportunities even after waiting for the negative conversion of sputum with TB treatment. However, the priority given to TB treatment should not result in missed opportunities for radical LC treatment, such as surgery or radiation chemotherapy. A previous Japanese retrospective study of 24 patients with TB-associated malignancies indicated that resumption of cancer treatment 2 months after completion of TB treatment may worsen the prognosis due to cancer progression [113]. Several papers have so far made suggestions for the timing of LC treatment for patients with concomitant TB. Ho et al. recommend that surgery and cytotoxic chemotherapy be administered 2–3 weeks after starting TB treatment and that molecularly targeted drugs be started while assessing drug interactions with anti-TB drugs and liver function [104]. The Japanese Society of Tuberculosis suggests that surgery should be performed 4 weeks after the start of TB treatment, and after confirmation of a negative smear, radiotherapy can be started at the same time as the TB treatment, and chemotherapy should be started 2–3 weeks after starting the TB treatment [114]. In our opinion, we offer the following treatment suggestions based on the previous reports [92,103,104,113,114] (Figure 1).
Figure 1.
Suggested algorithm for timing of lung cancer treatment in patients with comorbid sputum-smear/PCR positive active pulmonary tuberculosis.
3.4. Interactions between Drugs for Tuberculosis and Lung Cancer
When anti-Tb drugs and anti-cancer drugs are given simultaneously, attention should be paid to drug interactions between them. Among the anti-TB drugs, rifampicin is a potent inducer of cytochrome P450, which may attenuate the effect of some anti-cancer drugs [115]. In particular, rifampicin reduces the area under the concentration–time curve of most molecular-targeted drugs by 60–80%, and caution should be exercised when using them concomitantly [103,116]. The interactions between the molecular-targeted drugs and rifampicin, based on the drug’s package insert, are summarized in Table 2.
Table 2.
Drug interactions between molecularly targeted drugs and rifampicin.
| Main Target | Drugs | Metabolic Mediator | Decrease of AUC | Decrease of Cmax |
|---|---|---|---|---|
| EGFR | Gefitinib | CYP3A4 | 83% | 65% |
| Erlotinib | CYP3A4 | 69% | 39% | |
| Afatinib | P-glycoprotein | 34% | 22% | |
| Dacomitinib | CYP2D6 | No data | No data | |
| Osimertinib | CYP3A | 78% | 73% | |
| ALK | Alectinib | CYP3A4 | 73% | 51% |
| Lorlatinib | CYP3A, UGT1A4 | 85% | 76% | |
| Brigatinib | CYP2C8, CYP3A4 | 80% | 60% | |
| Ceritinib | CYP2C9, CYP3A4 | 70% | 44% | |
| ROS-1 | Crizotinib | CYP3A4 | 84% | 79% |
| ROS-1/NTRK | Entrectinib | CYP3A | 77% | 55% |
| BRAF | Dabrafenib | CYP2C8/9, CYP3A4 | 34% | 27% |
| Trametinib | CYP2B6, CYP3A4 | No data | No data | |
| MET | Tepotinib | CYP2C8/9, CYP3A4 | No data | No data |
| Capmatinib | CYP3A4 | 67% | 56% | |
| RET | Selpercatinib | CYP3A4 | 87% | 70% |
| KRAS | Sotorasib | CYP3A | 51% | 35% |
| HER2 | Trastuzumab | CYP3A | No data | No data |
Abbreviations: AUC, area under the concentration–time curve; Cmax, maximum plasma concentration; EGFR, epidermal growth factor receptor; ALK, anaplastic lymphoma kinase; ROS-1, c-ros oncogene 1; NTRK, neurotrophic receptor tyrosine kinase; BRAF, B-Raf proto-oncogene, serine/threonine kinase; MET, mesenchymal-epithelial transition; RET, rearranged during transfection; KRAS, v-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog; HER2, human epidermal growth factor type 2: CYP, cytochrome P450; UGT, UDP-glucuronosyl transferase.
Additionally, among cytotoxic anti-cancer drugs, etoposide, irinotecan, paclitaxel, docetaxel, and vinorelbine are metabolized by CYP3A4 and may be less effective when combined with RFP [103,117]. Therefore, during TB treatment, including rifampicin, the use of these antitumor drugs should be avoided as much as possible if alternative drugs are available. If the use of these antitumor drugs is absolutely necessary, switching to rifabutin, which has a weaker CYP induction effect, instead of rifampicin, should be considered as an option.
4. Conclusions
LC patients are often complicated by other respiratory diseases, with the management of which being problematic. This review article summarizes the current evidence and discusses future prospects for treatment strategies for LC patients complicated by IP, severe COPD, and TB.
Approximately 5–15% of LC patients have IP, and it is most important to select a treatment that is less likely to induce acute exacerbations of pre-existing IP. For first-line treatment of advanced NSCLC with comorbid IP, carboplatin plus nab-PTX is the treatment regimen with the most reported efficacy and safety. Although the safety of ICIs for NSCLC with IP is still controversial, it has the potential to be the only existing therapy with long-term survival.
The prevalence of COPD in LC patients is approximately 40–70%; the severity of COPD is considered an important prognostic factor in LC patients. However, even in the cases of low pulmonary function requiring home oxygen therapy, up to the first-line or second-line treatment may be considered. In pharmacotherapy for NSCLC with severe COPD, it is important to select agents that cause fewer pulmonary adverse events and cardiovascular burden. In addition, clinicians should not forget to treat COPD.
Active TB is noted in 2–5% of LC cases, whereas LC is noted in 1–2% of active TB cases. When treating LC patients with pharmacotherapy, especially ICIs, the risk of developing TB should always be kept in mind. Ideally, it may be desirable to precede TB treatment until the risk of TB infection has decreased, but this should not result in the loss of the opportunity for radical treatment of LC. In the treatment of LC patients with active TB, consider adding LC treatment after 2–3 weeks of prior TB treatment.
This review article summarizes the current evidence and discusses future prospects for treatment strategies for LC patients complicated by IP, severe COPD, and TB. However, large prospective studies on LC patients complicating these respiratory diseases are limited, and the evidence is insufficient. In particular, although the safety of ICIs for NSCLC with these complications is still controversial, it has the potential for long-term survival. It is particularly important to identify risk factors and biomarkers that predict exacerbation of pre-existing lung disease by ICIs. For appropriate patient selection, large studies are warranted in the future to identify the risk factors for ICIs in NSCLC patients with these complications.
Acknowledgments
We would like to thank the patients and their families.
Author Contributions
R.O.: Conceptualization, Investigation, Visualization, Writing (Original Draft). S.I.: Conceptualization, Investigation, Writing (Review and Editing). T.K.: Investigation, Writing (Review and Editing). S.S.: Investigation, Writing (Review and Editing). C.Y.: Investigation, Writing (Review and Editing). K.K.: Investigation, Writing (Review and Editing). A.M.: Investigation, Writing (Review and Editing). A.S.: Investigation, Writing (Review and Editing). T.B.: Investigation, Writing (Review and Editing). T.O.: Conceptualization, Investigation, Writing (Review and Editing). All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Ikeda S reports grants and personal fees from Chugai, grants and personal fees from AstraZeneca, personal fees from Ono, personal fees from Bristol Myers Squibb, personal fees from Pfizer, personal fees from Boehringer Ingelheim, personal fees from Eli Lilly, and personal fees from Taiho, outside the submitted work. Sekine A reports personal fees from AstraZeneca, personal fees from Ono, personal fees from Boehringer Ingelheim, and personal fees from Eli Lilly outside the submitted work. Baba T reports personal fees from AstraZeneca, personal fees from Boehringer Ingelheim, and personal fees from Daiichi-Sankyo outside the submitted work. Ogura T reports personal fees from Shionogi, personal fees from Nippon Boehringer Ingelheim, and personal fees from Eisai outside the submitted work. All remaining authors have declared no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Raghu G., Nyberg F., Morgan G. The epidemiology of interstitial lung disease and its association with lung cancer. Br. J. Cancer. 2004;91((Suppl. S2)):S3–S10. doi: 10.1038/sj.bjc.6602061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ozawa Y., Suda T., Naito T., Enomoto N., Hashimoto D., Fujisawa T., Nakamura Y., Inui N., Nakamura H., Chida K. Cumulative incidence of and predictive factors for lung cancer in IPF. Respirology. 2009;14:723–728. doi: 10.1111/j.1440-1843.2009.01547.x. [DOI] [PubMed] [Google Scholar]
- 3.Omori T., Tajiri M., Baba T., Ogura T., Iwasawa T., Okudela K., Takemura T., Oba M.S., Maehara T., Nakayama H., et al. Pulmonary Resection for Lung Cancer in Patients With Idiopathic Interstitial Pneumonia. Ann. Thorac. Surg. 2015;100:954–960. doi: 10.1016/j.athoracsur.2015.03.094. [DOI] [PubMed] [Google Scholar]
- 4.Kawahara T., Sakashita H., Suzuki T., Tateishi T., Miyazaki Y. Real world data of combined lung cancer and interstitial lung disease. J. Thorac. Dis. 2019;11:4144–4151. doi: 10.21037/jtd.2019.10.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Selman M., King T.E., Jr., Pardo A. Idiopathic Pulmonary Fibrosis: Prevailing and Evolving Hypotheses about Its Pathogenesis and Implications for Therapy. Ann. Intern. Med. 2001;134:136–151. doi: 10.7326/0003-4819-134-2-200101160-00015. [DOI] [PubMed] [Google Scholar]
- 6.Leslie K.O., Smith M.L. Idiopathic Pulmonary Fibrosis May Be a Disease of Recurrent, Tractional Injury to the Periphery of the Aging Lung: A Unifying Hypothesis Regarding Etiology and Pathogenesis. Arch. Pathol. Lab. Med. 2012;136:591–600. doi: 10.5858/arpa.2011-0511-OA. [DOI] [PubMed] [Google Scholar]
- 7.Demopoulos K., Arvanitis D.A., Vassilakis D.A., Siafakas N.M., Spandidos D.A. MYCL1, FHIT, SPARC, p16(INK4) and TP53 genes associated to lung cancer in idiopathic pulmonary fibrosis. J. Cell. Mol. Med. 2002;6:215–222. doi: 10.1111/j.1582-4934.2002.tb00188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uematsu K., Yoshimura A., Gemma A., Mochimaru H., Hosoya Y., Kunugi S., Matsuda K., Seike M., Kurimoto F., Takenaka K., et al. Aberrations in the fragile histidine triad (FHIT) gene in idiopathic pulmonary fibrosis. Cancer Res. 2001;61:8527–8533. [PubMed] [Google Scholar]
- 9.Natsuizaka M., Chiba H., Kuronuma K., Otsuka M., Kudo K., Mori M., Bando M., Sugiyama Y., Takahashi H. Epidemi-ologic survey of Japanese patients with idiopathic pulmonary fibrosis and investigation of ethnic differences. Am. J. Respir. Crit. Care Med. 2014;190:773–779. doi: 10.1164/rccm.201403-0566OC. [DOI] [PubMed] [Google Scholar]
- 10.Tomassetti S., Gurioli C., Ryu J.H., Decker P.A., Ravaglia C., Tantalocco P., Buccioli M., Piciucchi S., Sverzellati N., Dubini A., et al. The Impact of Lung Cancer on Survival of Idiopathic Pulmonary Fibrosis. Chest. 2015;147:157–164. doi: 10.1378/chest.14-0359. [DOI] [PubMed] [Google Scholar]
- 11.Khan K.A., Kennedy M.P., Moore E., Crush L., Prendeville S., Maher M.M., Burke L., Henry M.T. Radiological Characteristics, Histological Features and Clinical Outcomes of Lung Cancer Patients with Coexistent Idiopathic Pulmonary Fibrosis. Lung. 2014;193:71–77. doi: 10.1007/s00408-014-9664-8. [DOI] [PubMed] [Google Scholar]
- 12.Collard H.R., Ryerson C.J., Corte T.J., Jenkins G., Kondoh Y., Lederer D.J., Lee J.S., Maher T.M., Wells A.U., Antoniou K.M., et al. Acute Exacerbation of Idiopathic Pulmonary Fibrosis. An International Working Group Report. Am. J. Respir. Crit. Care Med. 2016;194:265–275. doi: 10.1164/rccm.201604-0801CI. [DOI] [PubMed] [Google Scholar]
- 13.Song J.W., Hong S.-B., Lim C.-M., Koh Y., Kim D.S. Acute exacerbation of idiopathic pulmonary fibrosis: Incidence, risk factors and outcome. Eur. Respir. J. 2011;37:356–363. doi: 10.1183/09031936.00159709. [DOI] [PubMed] [Google Scholar]
- 14.Azuma A., Nukiwa T., Tsuboi E., Suga M., Abe S., Nakata K., Taguchi Y., Nagai S., Itoh H., Ohi M., et al. Double-blind, Placebo-controlled Trial of Pirfenidone in Patients with Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2005;171:1040–1047. doi: 10.1164/rccm.200404-571OC. [DOI] [PubMed] [Google Scholar]
- 15.Taniguchi H., Ebina M., Kondoh Y., Ogura T., Azuma A., Suga M., Taguchi Y., Takahashi H., Nakata K., Sato A., et al. Pirfenidone in idiopathic pulmonary fibrosis. Eur. Respir. J. 2010;35:821–829. doi: 10.1183/09031936.00005209. [DOI] [PubMed] [Google Scholar]
- 16.Park I.-N., Kim D.S., Shim T.S., Lim C.-M., Lee S.D., Koh Y., Kim W.S., Kim W.D., Jang S.J., Colby T.V. Acute Exacerbation of Interstitial Pneumonia Other Than Idiopathic Pulmonary Fibrosis. Chest. 2007;132:214–220. doi: 10.1378/chest.07-0323. [DOI] [PubMed] [Google Scholar]
- 17.Kudoh S., Kato H., Nishiwaki Y., Fukuoka M., Nakata K., Ichinose Y., Tsuboi M., Yokota S., Nakagawa K., Suga M., et al. Interstitial lung disease in Japanese patients with lung cancer: A cohort and nested case-control study. Am. J. Respir. Crit. Care Med. 2008;177:1348–1357. doi: 10.1164/rccm.200710-1501OC. [DOI] [PubMed] [Google Scholar]
- 18.Minegishi Y., Sudoh J., Kuribayasi H., Mizutani H., Seike M., Azuma A., Yoshimura A., Kudoh S., Gemma A. The safety and efficacy of weekly paclitaxel in combination with carboplatin for advanced non-small cell lung cancer with idiopathic interstitial pneumonias. Lung Cancer. 2011;71:70–74. doi: 10.1016/j.lungcan.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe N., Taniguchi H., Kondoh Y., Kimura T., Kataoka K., Nishiyama O., Kondo M., Hasegawa Y. Efficacy of Chemotherapy for Advanced Non-Small Cell Lung Cancer with Idiopathic Pulmonary Fibrosis. Respiration. 2012;85:326–331. doi: 10.1159/000342046. [DOI] [PubMed] [Google Scholar]
- 20.Nakajima M., Yamamoto N., Hayashi K., Karube M., Ebner D.K., Takahashi W., Anzai M., Tsushima K., Tada Y., Tatsumi K., et al. Carbon-ion radiotherapy for non-small cell lung cancer with interstitial lung disease: A retrospective analysis. Radiat. Oncol. 2017;12:144. doi: 10.1186/s13014-017-0881-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rana J.N., Mumtaz S., Han I., Choi E.H. Formation of reactive species via high power microwave induced DNA damage and promoted intrinsic pathway-mediated apoptosis in lung cancer cells: An in vitro investigation. Fundam. Res. 2024 doi: 10.1016/j.fmre.2024.02.001. in press . [DOI] [Google Scholar]
- 22.Delaunois L.M. Mechanisms in pulmonary toxicology. Clin. Chest Med. 2004;25:1–14. doi: 10.1016/S0272-5231(03)00122-9. [DOI] [PubMed] [Google Scholar]
- 23.Kenmotsu H., Naito T., Kimura M., Ono A., Shukuya T., Nakamura Y., Tsuya A., Kaira K., Murakami H., Takahashi T., et al. The Risk of Cytotoxic Chemotherapy-Related Exacerbation of Interstitial Lung Disease with Lung Cancer. J. Thorac. Oncol. 2011;6:1242–1246. doi: 10.1097/JTO.0b013e318216ee6b. [DOI] [PubMed] [Google Scholar]
- 24.Enomoto Y., Inui N., Kato T., Baba T., Karayama M., Nakamura Y., Ogura T., Suda T. Low forced vital capacity predicts cytotoxic chemotherapy-associated acute exacerbation of interstitial lung disease in patients with lung cancer. Lung Cancer. 2016;96:63–67. doi: 10.1016/j.lungcan.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 25.Salisbury M.L., Xia M., Zhou Y., Murray S., Tayob N., Brown K.K., Wells A.U., Schmidt S.L., Martinez F.J., Flaherty K.R. Idiopathic Pulmonary Fibrosis: Gender-Age-Physiology Index Stage for Predicting Future Lung Function Decline. Chest. 2016;149:491–498. doi: 10.1378/chest.15-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kobayashi H., Omori S., Nakashima K., Wakuda K., Ono A., Kenmotsu H., Naito T., Murakami H., Endo M., Takahashi T. Modified GAP index for prediction of acute exacerbation of idiopathic pulmonary fibrosis in non-small cell lung cancer. Respirology. 2017;22:1379–1385. doi: 10.1111/resp.13075. [DOI] [PubMed] [Google Scholar]
- 27.Ogura T., Takigawa N., Tomii K., Kishi K., Inoue Y., Ichihara E., Homma S., Takahashi K., Akamatsu H., Ikeda S., et al. Summary of the Japanese Respiratory Society statement for the treatment of lung cancer with comorbid interstitial pneumonia. Respir. Investig. 2019;57:512–533. doi: 10.1016/j.resinv.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 28.Isono T., Kagiyama N., Takano K., Hosoda C., Nishida T., Kawate E., Kobayashi Y., Ishiguro T., Takaku Y., Kurashima K., et al. Outcome and risk factor of immune-related adverse events and pneumonitis in patients with advanced or postoperative recurrent non-small cell lung cancer treated with immune checkpoint inhibitors. Thorac. Cancer. 2020;12:153–164. doi: 10.1111/1759-7714.13736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tasaka Y., Honda T., Nishiyama N., Tsutsui T., Saito H., Watabe H., Shimaya K., Mochizuki A., Tsuyuki S., Kawahara T., et al. Non-inferior clinical outcomes of immune checkpoint inhibitors in non-small cell lung cancer patients with interstitial lung disease. Lung Cancer. 2021;155:120–126. doi: 10.1016/j.lungcan.2021.03.014. [DOI] [PubMed] [Google Scholar]
- 30.Shibaki R., Murakami S., Matsumoto Y., Yoshida T., Goto Y., Kanda S., Horinouchi H., Fujiwara Y., Yamamoto N., Kusumoto M., et al. Association of immune-related pneumonitis with the presence of preexisting interstitial lung disease in patients with non-small lung cancer receiving anti-programmed cell death 1 antibody. Cancer Immunol. Immunother. 2019;69:15–22. doi: 10.1007/s00262-019-02431-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsumoto K., Shiroyama T., Kuge T., Miyake K., Yamamoto Y., Yoneda M., Yamamoto M., Naito Y., Suga Y., Fukushima K., et al. Impact of treatment line on risks and benefits of immune checkpoint inhibitor in patients with advanced non-small cell lung cancer and interstitial lung disease: A systematic review and meta-analysis of cohort studies. Transl. Lung Cancer Res. 2022;11:1835–1846. doi: 10.21037/tlcr-22-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang M., Fan Y., Nie L., Wang G., Sun K., Cheng Y. Clinical Outcomes of Immune Checkpoint Inhibitor Therapy in Patients With Advanced Non-small Cell Lung Cancer and Preexisting Interstitial Lung Diseases: A Systematic Review and Me-ta-analysis. Chest. 2022;161:1675–1686. doi: 10.1016/j.chest.2021.12.656. [DOI] [PubMed] [Google Scholar]
- 33.Fujimoto D., Morimoto T., Ito J., Sato Y., Ito M., Teraoka S., Otsuka K., Nagata K., Nakagawa A., Tomii K. A pilot trial of nivolumab treatment for advanced non-small cell lung cancer patients with mild idiopathic interstitial pneumonia. Lung Cancer. 2017;111:1–5. doi: 10.1016/j.lungcan.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 34.Fujimoto D., Yomota M., Sekine A., Morita M., Morimoto T., Hosomi Y., Ogura T., Tomioka H., Tomii K. Nivolumab for advanced non-small cell lung cancer patients with mild idiopathic interstitial pneumonia: A multicenter, open-label single-arm phase II trial. Lung Cancer. 2019;134:274–278. doi: 10.1016/j.lungcan.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 35.Ikeda S., Kato T., Kenmotsu H., Ogura T., Iwasawa S., Sato Y., Harada T., Kubota K., Tokito T., Okamoto I., et al. A Phase 2 Study of Atezolizumab for Pretreated NSCLC With Idiopathic Interstitial Pneumonitis. J. Thorac. Oncol. 2020;15:1935–1942. doi: 10.1016/j.jtho.2020.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ando M., Okamoto I., Yamamoto N., Takeda K., Tamura K., Seto T., Ariyoshi Y., Fukuoka M. Predictive factors for inter-stitial lung disease, antitumor response, and survival in non-small-cell lung cancer patients treated with gefitinib. J. Clin. Oncol. 2006;24:2549–2556. doi: 10.1200/JCO.2005.04.9866. [DOI] [PubMed] [Google Scholar]
- 37.Fujimoto D., Tomii K., Otoshi T., Kawamura T., Tamai K., Takeshita J., Tanaka K., Matsumoto T., Monden K., Nagata K., et al. Preex-isting interstitial lung disease is inversely correlated to tumor epidermal growth factor receptor mutation in patients with lung adenocarcinoma. Lung Cancer. 2013;80:159–164. doi: 10.1016/j.lungcan.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 38.Honda T., Sakashita H., Masai K., Totsuka H., Motoi N., Kobayashi M., Akashi T., Mimaki S., Tsuchihara K., Chiku S., et al. Deleterious Pul-monary Surfactant System Gene Mutations in Lung Adenocarcinomas Associated With Usual Interstitial Pneumonia. JCO Precis. Oncol. 2018;2:1–24. doi: 10.1200/PO.17.00301. [DOI] [PubMed] [Google Scholar]
- 39.Fujimoto K., Ikeda S., Tabata E., Kaneko T., Sagawa S., Yamada C., Kumagai K., Fukushima T., Haga S., Watanabe M., et al. KRAS G12C Inhibitor as a Treatment Option for Non-Small-Cell Lung Cancer with Comorbid In-terstitial Pneumonia. Cancers. 2024;16:1327. doi: 10.3390/cancers16071327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kenmotsu H., Yoh K., Mori K., Ono A., Baba T., Fujiwara Y., Yamaguchi O., Ko R., Okamoto H., Yamamoto N., et al. Phase II study of nab-paclitaxel + carboplatin for patients with non-small-cell lung cancer and interstitial lung disease. Cancer Sci. 2019;110:3738–3745. doi: 10.1111/cas.14217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Asahina H., Oizumi S., Takamura K., Harada T., Harada M., Yokouchi H., Kanazawa K., Fujita Y., Kojima T., Sugaya F., et al. A prospective phase II study of carboplatin and nab-paclitaxel in patients with advanced non-small cell lung cancer and concomitant interstitial lung disease (HOT1302) Lung Cancer. 2019;138:65–71. doi: 10.1016/j.lungcan.2019.09.020. [DOI] [PubMed] [Google Scholar]
- 42.Fukuizumi A., Minegishi Y., Omori M., Atsumi K., Takano N., Hisakane K., Takahashi S., Kobayashi K., Sugano T., Takeuchi S., et al. Weekly paclitaxel in combination with carboplatin for advanced non-small-cell lung cancer complicated by idiopathic interstitial pneumonias: A single-arm phase II study. Int. J. Clin. Oncol. 2019;24:1543–1548. doi: 10.1007/s10147-019-01516-9. [DOI] [PubMed] [Google Scholar]
- 43.Sekine A., Satoh H., Baba T., Ikeda S., Okuda R., Shinohara T., Komatsu S., Hagiwara E., Iwasawa T., Ogura T., et al. Safety and efficacy of S-1 in combination with carboplatin in non-small cell lung cancer patients with interstitial lung disease: A pilot study. Cancer Chemother. Pharmacol. 2016;77:1245–1252. doi: 10.1007/s00280-016-3040-8. [DOI] [PubMed] [Google Scholar]
- 44.Hanibuchi M., Kakiuchi S., Atagi S., Ogushi F., Shimizu E., Haku T., Toyoda Y., Azuma M., Kondo M., Kawano H., et al. A multicenter, open-label, phase II trial of S-1 plus carboplatin in advanced non-small cell lung cancer patients with interstitial lung disease. Lung Cancer. 2018;125:93–99. doi: 10.1016/j.lungcan.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 45.Omori M., Minegishi Y., Uruga H., Fukuizumi A., Isobe K., Izumi S., Koyama R., Bando M., Sugiyama H., Takahashi K., et al. Carboplatin and weekly paclitaxel in combination with bevacizumab for the treatment of advanced non-small cell lung cancer complicated by idiopathic interstitial pneumonias: A feasibility study. Respir. Investig. 2023;61:625–631. doi: 10.1016/j.resinv.2023.06.002. [DOI] [PubMed] [Google Scholar]
- 46.Otsubo K., Kishimoto J., Ando M., Kenmotsu H., Minegishi Y., Horinouchi H., Kato T., Ichihara E., Kondo M., Atagi S., et al. Nintedanib plus chemotherapy for nonsmall cell lung cancer with idiopathic pulmonary fibrosis: A randomised phase 3 trial. Eur. Respir. J. 2022;60:2200380. doi: 10.1183/13993003.00380-2022. [DOI] [PubMed] [Google Scholar]
- 47.Hamada S., Ichiyasu H., Ikeda T., Inaba M., Kashiwabara K., Sadamatsu T., Sato N., Akaike K., Okabayashi H., Saruwatari K., et al. Protective effect of bevacizumab on chemothera-py-related acute exacerbation of interstitial lung disease in patients with advanced non-squamous non-small cell lung cancer. BMC Pulm. Med. 2019;19:72. doi: 10.1186/s12890-019-0838-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Enomoto Y., Kenmotsu H., Watanabe N., Baba T., Murakami H., Yoh K., Ogura T., Takahashi T., Goto K., Kato T. Efficacy and Safety of Combined Carboplatin, Paclitaxel, and Bevacizumab for Patients with Advanced Non-squamous Non-small Cell Lung Cancer with Pre-existing Interstitial Lung Disease: A Retrospective Multi-institutional Study. Anticancer Res. 2015;35:4259–4263. [PubMed] [Google Scholar]
- 49.Shimizu R., Fujimoto D., Kato R., Otoshi T., Kawamura T., Tamai K., Matsumoto T., Nagata K., Otsuka K., Nakagawa A., et al. The safety and efficacy of paclitaxel and carboplatin with or without bevacizumab for treating patients with advanced nonsquamous non-small cell lung cancer with interstitial lung disease. Cancer Chemother. Pharmacol. 2014;74:1159–1166. doi: 10.1007/s00280-014-2590-x. [DOI] [PubMed] [Google Scholar]
- 50.Minegishi Y., Gemma A., Homma S., Kishi K., Azuma A., Ogura T., Hamada N., Taniguchi H., Hattori N., Nishioka Y., et al. Acute exacerbation of idiopathic interstitial pneumonias related to chemotherapy for lung cancer: Nationwide surveillance in Japan. ERJ Open Res. 2020;6:00184-2019. doi: 10.1183/23120541.00184-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Watanabe N., Niho S., Kirita K., Umemura S., Matsumoto S., Yoh K., Ohmatsu H., Goto K. Second-line docetaxel for patients with platinum-refractory advanced non-small cell lung cancer and interstitial pneumonia. Cancer Chemother. Pharmacol. 2015;76:69–74. doi: 10.1007/s00280-015-2775-y. [DOI] [PubMed] [Google Scholar]
- 52.Kenmotsu H., Naito T., Mori K., Ko R., Ono A., Wakuda K., Imai H., Taira T., Murakami H., Endo M., et al. Effect of platinum-based chemotherapy for non-small cell lung cancer patients with interstitial lung disease. Cancer Chemother. Pharmacol. 2015;75:521–526. doi: 10.1007/s00280-014-2670-y. [DOI] [PubMed] [Google Scholar]
- 53.Kato M., Shukuya T., Takahashi F., Mori K., Suina K., Asao T., Kanemaru R., Honma Y., Muraki K., Sugano K., et al. Pemetrexed for advanced non-small cell lung cancer patients with interstitial lung disease. BMC Cancer. 2014;14:508. doi: 10.1186/1471-2407-14-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kanai O., Kim Y.H., Demura Y., Kanai M., Ito T., Fujita K., Yoshida H., Akai M., Mio T., Hirai T. Efficacy and safety of nivolumab in non-small cell lung cancer with preexisting interstitial lung disease. Thorac. Cancer. 2018;9:847–855. doi: 10.1111/1759-7714.12759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Richeldi L., Cottin V., du Bois R.M., Selman M., Kimura T., Bailes Z., Schlenker-Herceg R., Stowasser S., Brown K.K. Nintedanib in patients with idiopathic pulmonary fibrosis: Combined evidence from the TOMORROW and INPULSIS® trials. Respir. Med. 2016;113:74–79. doi: 10.1016/j.rmed.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 56.Iwata T., West Japan Oncology Group. Yoshino I., Yoshida S., Ikeda N., Tsuboi M., Asato Y., Katakami N., Sakamoto K., Yamashita Y., et al. A phase II trial evaluating the efficacy and safety of perioperative pirfenidone for prevention of acute exacerbation of idiopathic pulmonary fibrosis in lung cancer patients undergoing pulmonary resection: West Japan Oncology Group 6711 L (PEOPLE Study) Respir. Res. 2016;17:90. doi: 10.1186/s12931-016-0398-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Young R.P., Hopkins R.J., Christmas T., Black P.N., Metcalf P., Gamble G.D. COPD prevalence is increased in lung cancer, independent of age, sex and smoking history. Eur. Respir. J. 2009;34:380–386. doi: 10.1183/09031936.00144208. [DOI] [PubMed] [Google Scholar]
- 58.Izquierdo J.L., Resano P., El Hechem A., Almonacid C., Graziani D., Sánchez I.M. Impact of COPD in patients with lung cancer and advanced disease treated with chemotherapy and/or tyrosine kinase inhibitors. Int. J. Chronic Obstr. Pulm. Dis. 2014;9:1053–1058. doi: 10.2147/COPD.S68766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gao Y., Guan W., Liu Q., Wang H., Zhu Y., Chen R., Zhang G. Impact of COPD and emphysema on survival of patients with lung cancer: A meta-analysis of observational studies. Respirology. 2015;21:269–279. doi: 10.1111/resp.12661. [DOI] [PubMed] [Google Scholar]
- 60.Iachina M., Jakobsen E., Møller H., Lüchtenborg M., Mellemgaard A., Krasnik M., Green A. The Effect of Different Comorbidities on Survival of Non-small Cells Lung Cancer Patients. Lung. 2014;193:291–297. doi: 10.1007/s00408-014-9675-5. [DOI] [PubMed] [Google Scholar]
- 61.Kuo C.-H., Wu C.-Y., Lee K.-Y., Lin S.-M., Chung F.-T., Lo Y.-L., Liu C.-Y., Hsiung T.-C., Yang C.-T., Wu Y.-C. Chronic Obstructive Pulmonary Disease in Stage I Non-small Cell Lung Cancer That Underwent Anatomic Resection: The Role of a Recurrence Promoter. COPD J. Chronic Obstr. Pulm. Dis. 2014;11:407–413. doi: 10.3109/15412555.2013.838946. [DOI] [PubMed] [Google Scholar]
- 62.A Kiri V., Soriano J.B., Visick G., Fabbri L.M. Recent trends in lung cancer and its association with COPD: An analysis using the UK GP Research Database. Prim. Care Respir. J. 2009;19:57–61. doi: 10.4104/pcrj.2009.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang W., Dou S., Dong W., Xie M., Cui L., Zheng C., Xiao W. Impact of COPD on prognosis of lung cancer: From a perspective on disease heterogeneity. Int. J. Chronic Obstr. Pulm. Dis. 2018;13:3767–3776. doi: 10.2147/COPD.S168048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Omote N., Hashimoto N., Morise M., Sakamoto K., Miyazaki S., Ando A., Nakahara Y., Hasegawa Y. Impact of mild to moderate COPD on feasibility and prognosis in non-small cell lung cancer patients who received chemotherapy. Int. J. Chronic Obstr. Pulm. Dis. 2017;12:3541–3547. doi: 10.2147/COPD.S149456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dong W., Du Y., Ma S. Impact of chemotherapy in the prognosis of non-small-cell lung cancer patients with severe to very severe COPD. Int. J. Chronic Obstr. Pulm. Dis. 2018;13:3805–3812. doi: 10.2147/COPD.S182173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hayama M., Suzuki H., Shiroyama T., Tamiya M., Okamoto N., Tanaka A., Morishita N., Nishida T., Nishihara T., Hirashima T. Chemotherapy for patients with advanced lung cancer receiving long-term oxygen therapy. J. Thorac. Dis. 2016;8:116–123. doi: 10.3978/j.issn.2072-1439.2016.01.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Goffin J.R., Corriveau S., Tang G.H., Pond G.R. Management and outcomes of patients with chronic obstructive lung disease and lung cancer in a public healthcare system. PLoS ONE. 2021;16:e0251886. doi: 10.1371/journal.pone.0251886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Feary J.R., Rodrigues L.C., Smith C.J., Hubbard R.B., Gibson J.E. Prevalence of major comorbidities in subjects with COPD and incidence of myocardial infarction and stroke: A comprehensive analysis using data from primary care. Thorax. 2010;65:956–962. doi: 10.1136/thx.2009.128082. [DOI] [PubMed] [Google Scholar]
- 69.Tamiya A., Naito T., Miura S., Morii S., Tsuya A., Nakamura Y., Kaira K., Murakami H., Takahashi T., Yamamoto N., et al. Interstitial lung disease associated with docetaxel in patients with advanced non-small cell lung cancer. Anticancer Res. 2012;32:1103–1106. [PubMed] [Google Scholar]
- 70.Szentkereszty M., Komlósi Z.I., Szűcs G., Barna G., Tamási L., Losonczy G., Gálffy G. Effect of COPD on Inflammation, Lymphoid Functions and Progression-Free Survival during First-Line Chemotherapy in Advanced Non-small Cell Lung Cancer. Pathol. Oncol. Res. 2019;26:1117–1128. doi: 10.1007/s12253-019-00661-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhou J., Chao Y., Yao D., Ding N., Li J., Gao L., Zhang Y., Xu X., Zhou J., Halmos B., et al. Impact of chronic obstructive pulmonary disease on immune checkpoint inhibitor efficacy in advanced lung cancer and the potential prognostic factors. Transl. Lung Cancer Res. 2021;10:2148–2162. doi: 10.21037/tlcr-21-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shin S.H., Park H.Y., Im Y., Jung H.A., Sun J., Ahn J.S., Ahn M., Park K., Lee H.Y., Lee S. Improved treatment outcome of pembrolizumab in patients with nonsmall cell lung cancer and chronic obstructive pulmonary disease. Int. J. Cancer. 2019;145:2433–2439. doi: 10.1002/ijc.32235. [DOI] [PubMed] [Google Scholar]
- 73.Mark N.M., Kargl J., Busch S.E., Yang G.H.Y., Metz H.E., Zhang H., Hubbard J.J., Pipavath S.N.J., Madtes D.K., Houghton A.M. Chronic Obstructive Pulmonary Disease Alters Immune Cell Composition and Immune Checkpoint Inhibitor Efficacy in Non–Small Cell Lung Cancer. Am. J. Respir. Crit. Care Med. 2018;197:325–336. doi: 10.1164/rccm.201704-0795OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McKendry R.T., Spalluto C.M., Burke H., Nicholas B., Cellura D., Al-Shamkhani A., Staples K.J., Wilkinson T.M. Dysreg-ulation of Antiviral Function of CD8(+) T Cells in the Chronic Obstructive Pulmonary Disease Lung. Role of the PD-1-PD-L1 Axis. Am. J. Respir. Crit. Care Med. 2016;193:642–651. doi: 10.1164/rccm.201504-0782OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sul J., Blumenthal G.M., Jiang X., He K., Keegan P., Pazdur R. FDA Approval Summary: Pembrolizumab for the Treatment of Patients with Metastatic Non-Small Cell Lung Cancer Whose Tumors Express Programmed Death-Ligand 1. Oncologist. 2016;21:643–650. doi: 10.1634/theoncologist.2015-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Atchley W.T., Alvarez C., Saxena-Beem S., Schwartz T.A., Ishizawar R.C., Patel K.P., Rivera M.P. Immune Checkpoint Inhibitor-Related Pneumonitis in Lung Cancer: Real-World Incidence, Risk Factors, and Management Practices Across Six Health Care Centers in North Carolina. Chest. 2021;160:731–742. doi: 10.1016/j.chest.2021.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang K., Zhou C., Gao J., Yu P., Lin X., Xie X., Liu M., Zhang J., Xie Z., Cui F., et al. Treatment response and safety of immunotherapy for advanced non-small cell lung cancer with comorbid chronic obstructive pulmonary disease: A retrospective cohort study. Transl. Lung Cancer Res. 2022;11:2306–2317. doi: 10.21037/tlcr-22-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ikeda S., Kato T., Kenmotsu H., Sekine A., Baba T., Ogura T. Current Treatment Strategies for Non-Small-Cell Lung Cancer with Comorbid Interstitial Pneumonia. Cancers. 2021;13:3979. doi: 10.3390/cancers13163979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ajimizu H., Ozasa H., Sato S., Funazo T., Sakamori Y., Nomizo T., Kuninaga K., Ogimoto T., Hosoya K., Yamazoe M., et al. Survival impact of treatment for chronic obstructive pulmonary disease in patients with advanced non-small-cell lung cancer. Sci. Rep. 2021;11:23677. doi: 10.1038/s41598-021-03139-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wu C.Y., Hu H.Y., Pu C.Y., Huang N., Shen H.C., Li C.P., Chou Y.J. Pulmonary tuberculosis increases the risk of lung cancer: A population-based cohort study. Cancer. 2011;117:618–624. doi: 10.1002/cncr.25616. [DOI] [PubMed] [Google Scholar]
- 81.Hong S., Mok Y., Jeon C., Jee S.H., Samet J.M. Tuberculosis, smoking and risk for lung cancer incidence and mortality. Int. J. Cancer. 2016;139:2447–2455. doi: 10.1002/ijc.30384. [DOI] [PubMed] [Google Scholar]
- 82.Cheng M.P., Chakra C.N.A., Yansouni C.P., Cnossen S., Shrier I., Menzies D., Greenaway C. Risk of Active Tuberculosis in Patients with Cancer: A Systematic Review and Meta-Analysis. Clin. Infect. Dis. 2017;64:635–644. doi: 10.1093/cid/ciw838. [DOI] [PubMed] [Google Scholar]
- 83.Suzuki Y., Imokawa S., Sato J., Uto T., Suda T. Cumulative incidence of tuberculosis in lung cancer patients in Japan: A 6-year observational study. Respir. Investig. 2016;54:179–183. doi: 10.1016/j.resinv.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 84.Komatsu H., Ishizuka Y., Yoneda R. A study of coexistence of bronchogenic carcinoma and active pulmonary tuberculosis (author’s transl) Kekkaku. 1981;56:49–55. (In Japanese) [PubMed] [Google Scholar]
- 85.Ogawa N., Arai T., Inagaki K., Morita K., Yano M., Miyazawa H. A study on active pulmonary tuberculosis with coexistent lung carcinoma. Nihon Kyobu Rinsho. 1990;49:901–907. (In Japanese) [Google Scholar]
- 86.Kurasawa T., Takahashi M., Kuze F., Amitani R., Murayama T., Suzuki K., Kubo Y., Niimi A., Ikeda N., Nakatani K. A clinical study on coexistence of active pulmonary tuberculosis and lung cancer. Kekkaku. 1992;67:119–125. (In Japanese) [PubMed] [Google Scholar]
- 87.Watanabe A., Tokue Y., Takahashi H., Sato K., Nukiwa T., Honda Y., Fujimura S. Management of mycobacteriosis in general hospital without isolation ward for tuberculosis patients. Clinical study on pulmonary tuberculosis associated with lung cancer patients. Kekkaku. 1999;74:157–162. (In Japanese) [PubMed] [Google Scholar]
- 88.Tamura A., Hebisawa A., Masuda K., Shimada M., Ichikawa M., Kunogi M., Kaneko Y., Kawashima M., Suzuki J., Ariga H., et al. Coexisting lung cancer and active pulmonary mycobacteriosis. Nihon Kokyuki Gakkai Zasshi. 2007;45:382–393. (In Japanese) [PubMed] [Google Scholar]
- 89.Simonsen D.F., Farkas D.K., Søgaard M., Horsburgh C.R., Sørensen H.T., Thomsen R.W. Tuberculosis and risk of cancer: A Danish nationwide cohort study. Int. J. Tuberc. Lung Dis. 2014;18:1211–1219. doi: 10.5588/ijtld.14.0161. [DOI] [PubMed] [Google Scholar]
- 90.Jurado J.O., Alvarez I.B., Pasquinelli V., Martínez G.J., Quiroga M.F., Abbate E., Musella R.M., Chuluyan H.E., García V.E. Programmed Death (PD)-1:PD-Ligand 1/PD-Ligand 2 Pathway Inhibits T Cell Effector Functions during Human Tuberculosis. J. Immunol. 2008;181:116–125. doi: 10.4049/jimmunol.181.1.116. [DOI] [PubMed] [Google Scholar]
- 91.Alberg A.J., Brock M.V., Ford J.G., Samet J.M., Spivack S.D. Epidemiology of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143((Suppl. S5)):e1S–e29S. doi: 10.1378/chest.12-2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kim D.K., Lee S.W., Yoo C.-G., Kim Y.W., Han S.K., Shim Y.-S., Yim J.-J. Clinical Characteristics and Treatment Responses of Tuberculosis in Patients with Malignancy Receiving Anticancer Chemotherapy. Chest. 2005;128:2218–2222. doi: 10.1378/chest.128.4.2218. [DOI] [PubMed] [Google Scholar]
- 93.Huang S.-F., Li C.-P., Feng J.-Y., Chao Y., Su W.-J. Increased risk of tuberculosis after gastrectomy and chemotherapy in gastric cancer: A 7-year cohort study. Gastric Cancer. 2011;14:257–265. doi: 10.1007/s10120-011-0037-1. [DOI] [PubMed] [Google Scholar]
- 94.Tamura A. Tuberculosis and Lung Cancer. Kekkaku. 2016;91:17–25. [PubMed] [Google Scholar]
- 95.Barber D.L., Sakai S., Kudchadkar R.R., Fling S.P., Day T.A., Vergara J.A., Ashkin D., Cheng J.H., Lundgren L.M., Raabe V.N., et al. Tuberculosis following PD-1 blockade for cancer im-munotherapy. Sci. Transl. Med. 2019;11:eaat2702. doi: 10.1126/scitranslmed.aat2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fujita K., Terashima T., Mio T. Anti-PD1 Antibody Treatment and the Development of Acute Pulmonary Tuberculosis. J. Thorac. Oncol. 2016;11:2238–2240. doi: 10.1016/j.jtho.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 97.Takata S., Koh G., Han Y., Yoshida H., Shiroyama T., Takada H., Masuhiro K., Nasu S., Morita S., Tanaka A., et al. Paradoxical response in a patient with non-small cell lung cancer who received nivolumab followed by anti-Mycobacterium tuberculosis agents. J. Infect. Chemother. 2018;25:54–58. doi: 10.1016/j.jiac.2018.06.016. [DOI] [PubMed] [Google Scholar]
- 98.Tezera L.B., Bielecka M.K., Ogongo P., Walker N.F., Ellis M., Garay-Baquero D.J., Thomas K., Reichmann M.T., A Johnston D., Wilkinson K.A., et al. Anti-PD-1 immunotherapy leads to tuberculosis reactivation via dysregulation of TNF-α. eLife. 2020;9:e52668. doi: 10.7554/eLife.52668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fujita K., Yamamoto Y., Kanai O., Okamura M., Hashimoto M., Nakatani K., Sawai S., Mio T. Incidence of Active Tu-berculosis in Lung Cancer Patients Receiving Immune Checkpoint Inhibitors. Open Forum Infect Dis. 2020;7:ofaa126. doi: 10.1093/ofid/ofaa126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhu J., He Z., Liang D., Yu X., Qiu K., Wu J. Pulmonary tuberculosis associated with immune checkpoint inhibitors: A pharmacovigilance study. Thorax. 2022;77:721–723. doi: 10.1136/thoraxjnl-2021-217575. [DOI] [PubMed] [Google Scholar]
- 101.Leung C.C., Hui L., Lee R.S.Y., Lam T.H., Yew W.W., Hui D.S.C., Chan R.C.Y., Mok T.Y.W., Law W.S., Chang K.C., et al. Tuberculosis is associated with increased lung cancer mortality. Int. J. Tuberc. Lung Dis. 2013;17:687–692. doi: 10.5588/ijtld.12.0816. [DOI] [PubMed] [Google Scholar]
- 102.Parker C.S., Siracuse C.G., Litle V.R. Identifying lung cancer in patients with active pulmonary tuberculosis. J. Thorac. Dis. 2018;10((Suppl. S28)):S3392–S3397. doi: 10.21037/jtd.2018.07.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hirashima T., Tamura Y., Han Y., Hashimoto S., Tanaka A., Shiroyama T., Morishita N., Suzuki H., Okamoto N., Akada S., et al. Efficacy and safety of concurrent anti-Cancer and anti-tuberculosis chemotherapy in Cancer patients with active Mycobacterium tuberculosis: A retrospective study. BMC Cancer. 2018;18:975. doi: 10.1186/s12885-018-4889-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ho J.C.-M., Leung C.-C. Management of co-existent tuberculosis and lung cancer. Lung Cancer. 2018;122:83–87. doi: 10.1016/j.lungcan.2018.05.030. [DOI] [PubMed] [Google Scholar]
- 105.Zheng L., Yin J., Wang S., Jiang H., Hu P., Kang Z., Lv P., Li W., Cai C. Associated factors of co-existent pulmonary tu-berculosis and lung cancer: A case-control study. Eur. J. Clin. Investig. 2021;51:e13432. doi: 10.1111/eci.13432. [DOI] [PubMed] [Google Scholar]
- 106.Sun W., Zhang L., Liang J., Li X., Yang Y., Sun W., Hou J. Comparison of clinical and imaging features between pulmonary tuberculosis complicated with lung cancer and simple pulmonary tuberculosis: A systematic review and meta-analysis. Epidemiol. Infect. 2022;150:e43. doi: 10.1017/S0950268822000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dobler C.C., Cheung K., Nguyen J., Martin A. Risk of tuberculosis in patients with solid cancers and haematological ma-lignancies: A systematic review and meta-analysis. Eur. Respir. J. 2017;50:1700157. doi: 10.1183/13993003.00157-2017. [DOI] [PubMed] [Google Scholar]
- 108.Champiat S., Lambotte O., Barreau E., Belkhir R., Berdelou A., Carbonnel F., Cauquil C., Chanson P., Collins M., Durrbach A., et al. Management of immune checkpoint blockade dysimmune toxicities: A collaborative position paper. Ann. Oncol. 2015;27:559–574. doi: 10.1093/annonc/mdv623. [DOI] [PubMed] [Google Scholar]
- 109.Tamura A., Ikeda M., Shimada M., Yamane A. Tuberculosis during lung cancer treatment-A case series. J. Infect. Chemother. 2021;28:339–342. doi: 10.1016/j.jiac.2021.10.017. [DOI] [PubMed] [Google Scholar]
- 110.Mori T., Harada N., Higuchi K., Sekiya Y., Uchimura K., Shimao T. Waning of the specific interferon-gamma response after years of tuberculosis infection. Int. J. Tuberc. Lung Dis. 2007;11:1021–1025. [PubMed] [Google Scholar]
- 111.Tamura A., Fukami T., Hebisawa A., Takahashi F. Recent trends in the incidence of latent tuberculosis infection in Japanese patients with lung cancer: A small retrospective study. J. Infect. Chemother. 2019;26:315–317. doi: 10.1016/j.jiac.2019.10.018. [DOI] [PubMed] [Google Scholar]
- 112.Ye M.-F., Su S., Huang Z.-H., Zou J.-J., Su D.-H., Chen X.-H., Zeng L.-F., Liao W.-X., Huang H.-Y., Zeng Y.-Y., et al. Efficacy and safety of concurrent anti-tuberculosis treatment and chemotherapy in lung cancer patients with co-existent tuberculosis. Ann. Transl. Med. 2020;8:1143. doi: 10.21037/atm-20-5964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fujita T., Endo M., Gu Y., Sato T., Ohmagari N. Mycobacterium tuberculosis infection in cancer patients at a tertiary care cancer center in Japan. J. Infect. Chemother. 2014;20:213–216. doi: 10.1016/j.jiac.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 114.Tamura A. Cancer and Tuberculosis/Nontuberculous Mycobacteriosis. Kekkaku. 2022;97:13–20. (In Japanese) [Google Scholar]
- 115.Kajosaari L.I., Laitila J., Neuvonen P.J., Backman J.T. Metabolism of Repaglinide by CYP2C8 and CYP3A4 in vitro: Effect of Fibrates and Rifampicin. Basic Clin. Pharmacol. Toxicol. 2005;97:249–256. doi: 10.1111/j.1742-7843.2005.pto_157.x. [DOI] [PubMed] [Google Scholar]
- 116.Rakhit A., Pantze M.P., Fettner S., Jones H.M., Charoin J.E., Riek M., Lum B.L., Hamilton M. The effects of CYP3A4 inhi-bition on erlotinib pharmacokinetics: Computer-based simulation (SimCYP) predicts in vivo metabolic inhibition. Eur. J. Clin. Pharmacol. 2008;64:31–41. doi: 10.1007/s00228-007-0396-z. [DOI] [PubMed] [Google Scholar]
- 117.Bragalone D.L. Drug Information Handbook for Oncology. 11th ed. Lexi-Comp; Hudson, OH, USA: 2013. [Google Scholar]