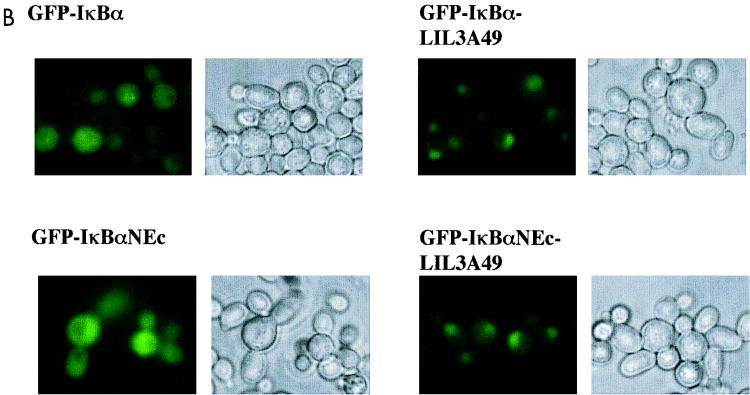
FIG. 3.
Point mutational analysis of a putative N-terminal NES in IκBα. (A) Schematic representation of the N-terminal and first ankyrin domains of IκBα. The sequence shown above the N-terminal domain includes residues 45 to 58 of human IκBα, whose deletion in NΔ55 (Fig. 2) makes IκBα constitutively nuclear. The indicated residues were changed to alanines to generate the mutant referred to as LIL3A49 (the first leucine is residue 49). The sequence above the ankyrin domain shows another leucine- or isoleucine-rich motif comprised of residues 76 to 85. Mutations LI2A78 and LLII4A78 alter the first leucine and isoleucine, or both leucines and isoleucines, respectively. Mutations were in the context of full-length (F.L.) WT IκBα or a derivative mutated at the C-terminal NES (NEc). Columns on the right summarize the subcellular location of mutants. C, either cytoplasm or cytoplasm plus nuclear; N, nuclear localization. Representative data in yeast and mammalian cells are shown in panels B and C, respectively. (B) Subcellular distribution of GFP-IκBα derivatives indicated in CRM1+ yeast strain. Data shown are representative of at least three independent experiments. (C) Subcellular distribution of GFP-IκBα derivatives in BOSC 23 mammalian cells visualized after transient transfection with appropriate expression vectors as indicated. DAPI staining was used to visualize nuclei; GFP fluorescence is present in a subset of cells because not all cells pick up transfected DNA.