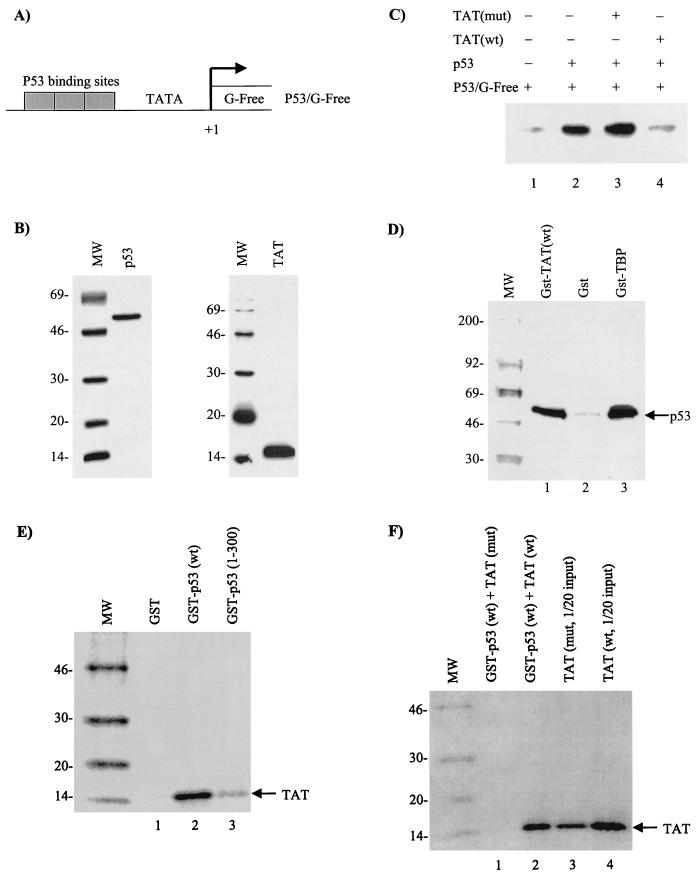
FIG. 5.
In vitro analysis of Tat on P53-activated transcription. In vitro transcription assays were performed with a supercoiled G-free cassette DNA (P53/G-Free), CEM (12D7) whole-cell extract, and purified p53 and Tat proteins. (A) Diagram of the reporter G-Free plasmid. (B) Coomassie blue stain of p53 and Tat proteins used for in vitro transcription assays. (C) In vitro transcription with p53 (lane 2 was treated with monoclonal antibody PAb 421 to increase DNA binding) in the presence (+) or absence (−) of either mutant (lane 3, Tat 41 mutant [25]) (mut) or wild-type (lane 4) (wt) Tat. Neither wild-type nor mutant Tat protein alone activated P53/G-Free transcription (data not shown). (D) In vitro binding of GST-TBP (positive control), GST, or GST-Tat to 35S-labeled p53 (TNT; Promega). Samples were bound overnight and washed the next day, and bound p53 proteins were run on a 4 to 20% Tris-glycine gel, dried, and exposed to a PhosphorImager cassette. (E) Binding of 35S-labeled Tat to GST-p53 wild type or a mutant containing p53 residues 1 to 300. As previously reported (27), Tat binds to the C-terminal domain of p53. (F) Binding of either the wild-type or a 41 mutant of Tat to GST-p53. The experiments shown in panels E and F were performed in the presence of 50 μg of ethidium bromide/ml (to eliminate nonspecific binding to nucleic acids) and washed with TNE150 plus 0.1% NP-40 buffer prior to being loaded on SDS-PAGE.