Abstract
Background
There is limited evidence suggesting that osteoporosis might exacerbate depressive symptoms, while more studies demonstrate that depression negatively affects bone density and increases fracture risk.
Aims
To explore the relationship between major depressive disorder (MDD) and fracture risk.
Methods
We conducted a nested case-control analysis (32 670 patients with fracture and 397 017 individuals without fracture) and a matched cohort analysis (16 496 patients with MDD and 435 492 individuals without MDD) in the same prospective UK Biobank data set. Further, we investigated the shared genetic architecture between MDD and fracture with linkage disequilibrium score regression and the MiXeR statistical tools. We used the conditional/conjunctional false discovery rate approach to identify the specific shared loci. We calculated the weighted genetic risk score for individuals in the UK Biobank and logistic regression was used to confirm the association observed in the prospective study.
Results
We found that MDD was associated with a 14% increase in fracture risk (hazard ratio (HR) 1.14, 95% CI 1.14 to 1.15, p<0.001) in the nested case-control analysis, while fracture was associated with a 72% increase in MDD risk (HR 1.72, 95% CI 1.64 to 1.79, p<0.001) in the matched cohort analysis, suggesting a longitudinal and bidirectional relationship. Further, genetic summary data suggested a genetic overlap between MDD and fracture. Specifically, we identified four shared genomic loci, with the top signal (rs7554101) near SGIP1. The protein encoded by SGIP1 is involved in cannabinoid receptor type 1 signalling. We found that genetically predicted MDD was associated with a higher risk of fracture and vice versa. In addition, we found that the higher expression level of SGIP1 in the spinal cord and muscle was associated with an increased risk of fracture and MDD.
Conclusions
The genetic pleiotropy between MDD and fracture highlights the bidirectional association observed in the epidemiological analysis. The shared genetic components (such as SGIP1) between the diseases suggest that modulating the endocannabinoid system could be a potential therapeutic strategy for both MDD and bone loss.
Keywords: Depressive Disorder, Major; Case-Control Studies; Cohort Studies; Observation; Genome-Wide Association Study
WHAT IS ALREADY KNOWN ON THIS TOPIC.
Limited evidence suggests that osteoporosis might exacerbate depressive symptoms, while more studies show that depression negatively affects bone density and increases fracture risk. However, the Mendelian randomisation approach using the genome-wide association study summary data reported that genetic predisposition towards depression had no casual association with bone mineral density and fracture.
WHAT THIS STUDY ADDS
We demonstrated that major depressive disorder (MDD) was associated with the risk of subsequent fractures. With the same cohort but different time series data, we observed a higher risk of MDD following the occurrence of a fracture. Further analysis using genetic data revealed a genetic overlap between MDD and fracture. Specifically, we identified four shared genomic loci, with the top signal (rs7554101) near SGIP1. We found that genetically predicted MDD was associated with a higher risk of fracture and vice versa. In addition, we found that the higher expression level of SGIP1 in the spinal cord and muscle was associated with an increased risk of fracture and MDD.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Our findings suggest that concurrent management of both diseases should be considered. The genetic contribution to both traits, highlighted by the shared loci (such asSGIP1), suggests that modulating the endocannabinoid system could be a potential therapeutic strategy for both diseases.
Introduction
Major depressive disorder (MDD), also known as clinical depression, is a chronic mood disorder characterised by a persistent feeling of sadness and loss of interest in previously enjoyed activities. It is a leading cause of disability, with incident cases increasing from 172 million in 1990 to 258 million in 2017.1 The lifetime prevalence of MDD is highest in individuals aged 18–29 years (21.0%), followed by those aged 30–44 years (16.6%) and 45–59 years (10.9%).2 MDD can lead to various complications, including cognitive changes,3 dementia,4 weight gain and obesity,5 diabetes6 and osteoporosis.7 Osteoporosis is a common systemic skeletal disease characterised by reduced bone mass and impaired bone quality,8 leading to increased bone fragility and a higher risk of low-trauma fracture, especially in women and older individuals.
Genome-wide association studies (GWAS) identified many genetic loci associated with depression.9 For example, a large GWAS meta-analysis conducted by the Psychiatric Genomics Consortium identified 44 risk variants for MDD.10 The heritability of MDD was estimated to be between 20% and 50%,11 indicating a substantial genetic contribution to its development. Similarly, GWAS have identified various genetic variants associated with fracture risk, particularly those involved in bone metabolism and mineral density.12 Osteoporotic fractures showed a substantial genetic component, with the extent of heritability varying depending on the specific bone site. Twin studies indicated that approximately 50% of the risk for non-vertebral fracture was attributable to genetic factors, while the heritability estimate for vertebral fracture was lower at around 24%.13 14
A previous study showed an association between depression and an increased risk of bone loss and fracture.15 An updated meta-analysis suggested individuals with MDD were more likely to experience low bone mineral density (BMD) in areas such as the spine, total hip and femoral neck, particularly in adults and women.16 A longitudinal study of 4224 older Australian men showed that past and present depression were both associated with a modest increase in the risk of incident fractures.17 On the other hand, limited findings suggested that osteoporosis might exacerbate depressive symptoms.18 19 A study of age-matched individuals with (296 women) and without (590 women) fracture demonstrated that fracture was associated with an increased risk of depression in older women.20 However, the Mendelian randomisation approach using the GWAS summary data reported that genetic predisposition towards depression had no casual association with BMD and fracture.21
In this study, using large-scale prospective biobank data, we first conducted a nested case-control study to investigate the association between MDD and fracture. Additionally, we carried out a matched cohort study to investigate the effect of fracture on MDD. Further, by integrating the genetic summary data for both MDD and fracture, we examined whether there are shared genetic architecture components between MDD and fracture, and assessed whether the observed relationship could be explained, at least partially, by genetic architecture.
Methods
Study participants
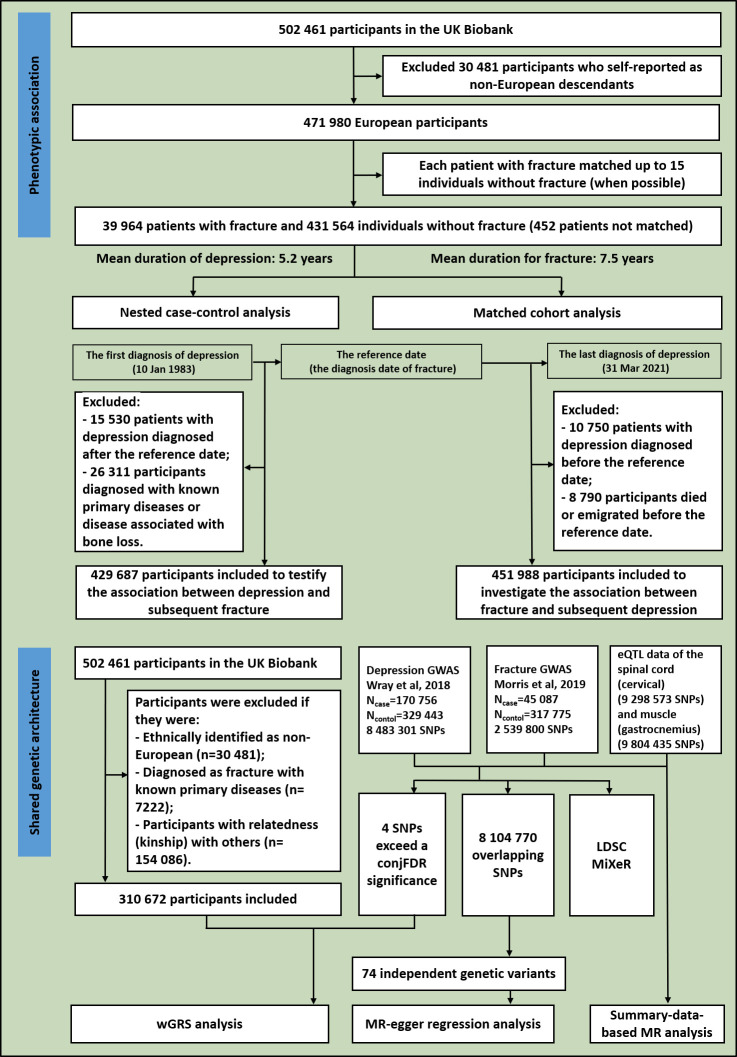
Similar to previous studies,22–24 the UK Biobank data were used in this study (application #41376). We identified the individuals with MDD and fracture using the International Classification of Diseases (ICD) codes. The detailed information on the field ID and the codes for data extraction from the UK Biobank are listed in online supplemental table 1. Among the 502 461 participants, 30 481 who self-reported as non-European descendants were excluded. The remaining 471 980 participants were followed until death, emigration or the latest diagnosis of MDD in the cohort (31 March 2021), whichever occurred first (figure 1). Within this nationwide study base, we identified 39 964 participants with a diagnosed fracture. Each patient with a fracture had a diagnosis date (ie, the reference date), which was used to calculate their age at the onset of the fracture. Among the participants who were free of fracture, we selected up to 15 age-matching participants (when possible). Finally, 431 564 participants free of fracture were individually matched, with 452 participants with fracture who were not matched (figure 1). Among the 39 964 participants with fracture, the matched participants free of fracture followed a varied pattern, with a peak at 10 matching participants, where 8670 participants with fracture fell within this category (online supplemental figure 1).
Figure 1.
The overall study design: flowchart of inclusion and exclusion in the phenotypic and genetic studies. conjFDR, conjunctional false discovery rate; eQTL, expression quantitative trait loci; GWAS, genome-wide association studies; LDSC, linkage disequilibrium score regression; MR, Mendelian randomisation; SNPs, single nucleotide polymorphisms; wGRS, weighted genetic risk score.
gpsych-2023-101418supp001.pdf (792.8KB, pdf)
Nested case-control analysis
We conducted a nested case-control analysis to assess the association between MDD and subsequent fracture with the incidence of fracture as the endpoint and the diagnosis date of fracture as the reference date, ensuring the validity of a full prospective cohort analysis.25 We ascertained the date of a first-ever diagnosis of MDD (10 January 1983). We excluded 15 530 patients with MDD diagnosed after the reference date and 26 311 participants diagnosed with a fracture with known primary diseases or diagnosed with diseases associated with bone loss (online supplemental table 2), remaining 429 687 participants (10 200 patients with MDD and 419 487 age-matched individuals without MDD) (figure 1). We also conducted stratified analysis by sex and by age group. There were 199 738 men and 229 949 women (online supplemental table 3). The population was divided into three age groups: ‘young adults’ (age ≤44 years, n=42 321), ‘middle-aged adults’ (age 45–59 years, n=197 980) and ‘older adults’ (age ≥60 years, n=189 386) (online supplemental table 4). We derived ORs and 95% CIs from the logistic regression by comparing patient cases with controls. The estimates should be interchangeably interpreted as the relative risk of fracture among patients with MDD.25 The estimates were conditioned on the factors impacting bone metabolism, such as age (reference age), body mass index (BMI), smoking, alcohol consumption frequency, employment status (paid employment, self-employed, others or unknown), education (college degree or below or unknown), physical activity, sleep duration, grip strength (right), falls, BMD and antidepressant medication in model 1. Individuals treated with any antidepressant medication including citalopram, escitalopram oxalate, fluoxetine, fluvoxamine, paroxetine, sertraline, Cymbalta, Elavil, tofranil, nortriptyline, Sinequan, Marplan, Nardil and Parnate were recorded as having received medical treatment. We further adjusted for cannabis intake as model 2.
Matched cohort analysis
We assessed the association between fracture and subsequent MDD using a matched cohort design (figure 1). In this analysis, we excluded 10 750 participants (1924 patients with fracture) who were diagnosed with MDD before the reference date and 8790 participants (11 patients with fracture) who died or emigrated before the reference date (figure 1). Prospective follow-up of the remaining 451 988 participants commenced from the reference date until the earliest of the following events: (1) diagnosis of MDD; (2) death; (3) emigration; (4) end of the study, defined as the diagnose date of the last patient with MDD (31 March 2021). We also conducted stratified analysis by sex and by age group. There were 205 249 men and 246 739 women (online supplemental table 3). Additionally, the population was divided into three age groups: ‘young adults’ (age ≤44 years, n=44 590), ‘middle-aged adults’ (age 45–59 years, n=208 889) and ‘older adults’ (age ≥60 years, n=198 509)(online supplemental table 4). We calculated the hazard ratio (HR) of MDD and 95% CI by using the stratified Cox proportional hazards regression comparing MDD cases and controls. In the basic model, we adjusted for the clinical risk factors including reference age, BMI, smoking, alcohol consumption frequency, employment status, education, physical activity, sleep duration, grip strength (right) and other mental disorders (mental and behavioural disorders, schizophrenia, schizotypal and delusional disorders, anxiety disorders, reaction to severe stress and adjustment disorders) using ICD codes26 (detailed information is listed in online supplemental table 1) (model 1). We further adjusted for cannabis intake as model 2.
Infer the shared genetics
The summary-statistic data for MDD were obtained from the most recent meta-analysis of GWAS of 33 cohorts from the Psychiatric Genomics Consortium,10 including 170 756 MDD cases and 329 443 controls analysed for 8 483 301 genetic variants. The summary-statistic data for fracture (45 087 cases and 317 775 controls) were extracted from another recent comprehensive study that investigated the genetic determinants of BMD and fracture12 (figure 1).
Using these summary-statistic GWAS data, we performed a genome-wide genetic correlation analysis between MDD and fracture by using linkage disequilibrium score regression (LDSC).27 We used MiXeR (V.1.2.0), an open-source software package available on GitHub (https://github.com/precimed/mixer), to compute the polygenic overlap between MDD and fracture, independent of genetic correlation.28 MiXeR models the additive genetic effects as a combination of four components, representing null single nucleotide polymorphisms (SNPs) in both traits (π0), SNPs with a specific effect on either MDD (π1) or fracture (π2) and SNPs with non-zero effect on both traits (π12). The degree of similarity between the two traits was estimated using the Dice coefficient, which was calculated as 2π12/(π1 + π2+2π12).28 To provide a visual pattern of pleiotropic enrichment between the two phenotypes, we generated conditional quantile-quantile (Q-Q) plots conditioning fracture on MDD and vice versa at various thresholds (p<0.1, p<0.01 and p<0.001).29
We used the conditional/conjunctional false discovery rate approach (https://github.com/precimed/pleiofdr) to identify the specific shared loci between MDD and fracture risk.30 To identify SNPs linked to one trait given associations with another, we used the conditional false discovery rate (condFDR) method with a significance cut-off of less than 0.01. We designated condFDR for fracture given associations with MDD as condFDR (fracture|MDD) and vice versa. The conjunctional FDR (conjFDR) method was applied to determine SNPs that were jointly associated with MDD and fracture. After conducting the condFDR procedure for both traits, the conjFDR analysis reported the loci that exceeded a condFDR significance threshold for two traits simultaneously (the maximum between the condFDRs for both traits), with the significance level set at conjFDR<0.05.
We constructed the weighted genetic risk score (wGRS) for individuals in the UK Biobank data set using a linear combination of the selected SNPs weighted by their β coefficients on MDD/fracture: wGRS=β1×SNP1 + β2×SNP2 + … + βn×SNPn, where n represents the number of instrumental variables. In the wGRS analysis, we excluded participants who met the following criteria: (1) ethnically identified as non-European (n=30 481); (2) diagnosed with a fracture with known primary diseases (n=7222); (3) participants with kinship relations with others (n=154 086). This left a cohort of 310 672 participants, including 24 376 patients with fracture and 17 158 patients with MDD (figure 1). We selected SNPs exceeding a conjFDR significance for MDD and fracture as instrumental variables (n=4) (figure 1). Next, logistic regression analyses were performed to analyse the association between the wGRS and fracture/MDD, adjusted for sex and age.
In order to verify shared loci, we merged the two summary data sets for MDD and fracture, resulting in 8 104 770 overlapping SNPs. Among these, 4425 SNPs were genome-wide significant for MDD (figure 1). The significance level for the GWAS of MDD or fracture in the summary data sets was set at the traditional genome-wide significance threshold of p<5×10-8. We applied linkage disequilibrium clumping based on r2<0.1 in a 500 kb window, resulting in 74 independent genetic variants (figure 1 and online supplemental table 5). We regressed the effects of these 74 SNPs for the two traits to highlight the overall effect of MDD on fracture by Mendelian randomisation-egger regression with the ‘grs.summary’ function in the R package ‘gtx’ (http://www2.uaem.mx/r-mirror/web/packages/gtx/gtx.pdf).
We employed the summary-data-based Mendelian randomisation (SMR) method developed by colleagues31 to test the association between gene expression levels and both MDD and fracture using summary-level data from GWAS and expression quantitative trait loci (eQTL) data of the spinal cord (cervical, 9 298 573 SNPs included) and muscle (gastrocnemius, 9 804 435 SNPs included) from the GTEx database (release V.8) (https://www.gtexportal.org/home/)32 (figure 1).
Gene mapping and enrichment analysis
We aligned the four candidate SNPs with genes using the following two strategies: (1) aligning SNPs to genes based on physical proximity, and (2) mapping SNPs to the genes whose expression level is influenced by allelic variation at the SNP level with the eQTL data from whole blood sourced from eQTLGen (a meta-analysis of 14 115 samples).33 We used GENE2FUNC as one of the core processes of functional mapping and annotation34 for the enrichment of differentially expressed gene sets in a specific tissue compared with all other tissue types. In addition, independent significant SNPs were also linked to the GWAS catalogue35 to provide insight into previously reported associations of the SNPs in the risk loci with various phenotypes.
Results
Longitudinal phenotypic association between MDD and fracture
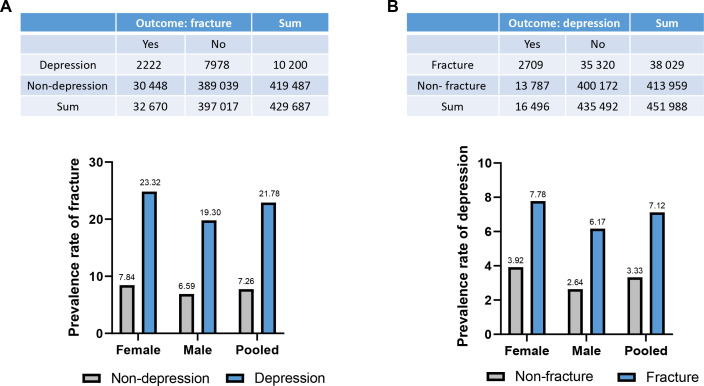
First, we examined the association between MDD and subsequent fracture in a nested case-control study. The nested case-control analysis (ie, assessing the incidence of MDD before the fracture diagnosis/matching date) is, by design, equivalent to a prospective analysis using the full cohort.25 We identified 32 670 (7.60%) participants with fracture among the 429 687 UK Biobank participants (figure 1 and online supplemental table 6). Notably, patients with MDD exhibited a higher prevalence of fractures compared with those without MDD (χ2=2988.82, p<0.001), with 2222 (21.78%) fractures occurring in individuals with MDD and 30 448 (7.26%) in those without (figure 2A, online supplemental table 3 and online supplemental table 6). The prevalence of fractures was higher in MDD patients than in those without MDD among both female and male participants (figure 2A and online supplemental table 3). Interestingly, female patients with MDD have a higher prevalence of fracture than their male counterparts (23.32% vs 19.30%, χ2=22.63, p<0.001) (figure 2A). Patients with MDD across all age groups exhibited a significantly higher prevalence of fracture (young adults: 17.82%, middle-aged adults: 20.79% and older adults: 23.55%) compared with individuals without MDD (5.28%, 6.31% and 8.70%, respectively) (all p<0.001) (online supplemental figure 2A and online supplemental table 4). Further, in the basic model of the logistic regression analysis (model 1, adjusted for reference age, BMI, smoking, alcohol consumption frequency, employment status, education, physical activity, sleep duration and grip strength), MDD was associated with a 14% increased risk of subsequent fracture (95% CI 14% to 15%, p<0.001; table 1), and it was found to be associated with 15% and 13% increase in fracture risk in women and men, respectively (women 95% CI 14% to 16%, p<0.001; men: 95% CI 12% to 14%, p<0.001). The results were similar when cannabis intake was included as a covariate (model 2) in pooled, female and male samples, respectively (table 1).
Figure 2.
The prevalence rates in men, women and pooled samples. (A) The prevalence rates of fracture in samples with or without MDD. (B) The prevalence rates of MDD in samples with or without fracture. MDD, major depressive disorder.
Table 1.
Longitudinally bidirectional association between MDD and fracture
| OR (95% CI)* | P value* | OR (95% CI)† | P value† | |
| Nested case-control design to assess the association of MDD with subsequent fracture | ||||
| Pooled | 1.14 (1.14 to 1.15) | <0.001 | 1.13 (1.12 to 1.14) | <0.001 |
| Female | 1.15 (1.14 to 1.16) | <0.001 | 1.15 (1.13 to 1.16) | <0.001 |
| Male | 1.13 (1.12 to 1.14) | <0.001 | 1.09 (1.08 to 1.11) | <0.001 |
| HR (95% CI)‡ | P value‡ | HR (95% CI)§ | P value§ | |
| Matched cohort design to assess the association of fracture with subsequent MDD | ||||
| Pooled | 1.72 (1.64 to 1.79) | <0.001 | 1.80 (1.64 to 1.98) | <0.001 |
| Female | 1.78 (1.69 to 1.88) | <0.001 | 1.98 (1.78 to 2.21) | <0.001 |
| Male | 1.62 (1.51 to 1.75) | <0.001 | 1.42 (1.19 to 1.70) | <0.001 |
*Model 1 adjusted for reference age, BMI, smoking, alcohol consumption frequency, employment status, education, physical activity, sleep duration, grip strength, falls, BMD and antidepressant medication.
†Model 2 adjusted for *+cannabis intake.
‡Model 1 adjusted for reference age, BMI, smoking, alcohol consumption frequency, employment status, education, physical activity, sleep duration, grip strength and pre-existing psychiatric disorder.
§Model 2 adjusted for ‡+cannabis intake.
BMD, bone mineral density; BMI, body mass index; HR, hazard ratio; MDD, major depressive disorder.
Second, we assessed the association between fracture and subsequent MDD in a matched cohort study (ie, addressing the incidence of MDD after the fracture diagnosis/matching date). During the follow-up (from the matching date to 31 March 2021), we identified 16 496 (3.65%) participants with MDD among the 451 988 UK Biobank participants (figure 1 and online supplemental table 6). Notably, individuals with fracture exhibited a higher prevalence of MDD compared with those without fracture, with 2709 (7.12%) occurrences in participants with fracture and 13 787 (3.33%) in those without (χ2=1423.88, p<0.001) (figure 2B, online supplemental table 3 and online supplemental table 6). Furthermore, female patients with fractures had a higher prevalence of MDD than their male counterparts (7.78% vs 6.17%, χ2=36.11, p<0.001) (figure 2B and online supplemental table 3). Across all age groups, patients with fractures experienced higher rates of MDD compared with their respective counterparts without fractures (young adults: 8.06% vs 3.62%, middle-aged adults: 7.75% vs 3.39% and older adults: 6.52% vs 3.19%, respectively) (online supplemental figure 2B and online supplemental table 4). After adjusting for the potential confounding effects of reference age, BMI, smoking, alcohol consumption frequency, employment status, education, physical activity, sleep duration, grip strength and previous diagnoses of psychiatric disorders (model 1), we observed that fracture was associated with a high risk of MDD with an HR of 1.72 (95% CI 1.64 to 1.79, p<0.001) in pooled samples, 1.78 (95% CI 1.69 to 1.88) in women and 1.62 (95% CI 1.51 to 1.75) in men, respectively (table 1). Even after additional control for cannabis intake (model 2), we still observed an increased risk of MDD in fracture (HR=1.80, 95% CI 1.64 to 1.98, p<0.001) (table 1).
Genetic overlap between MDD and fracture
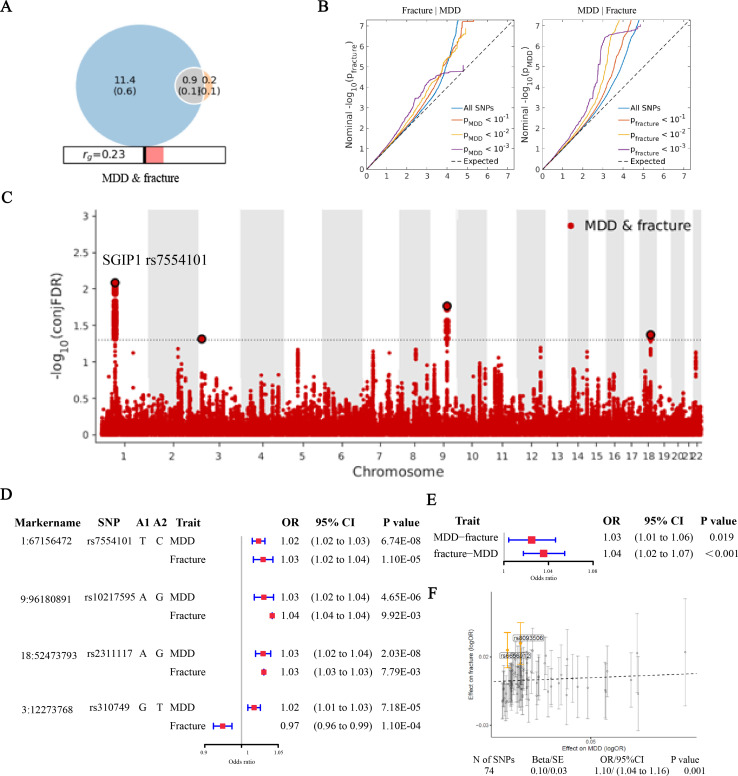
We calculated the genetic correlation between MDD and fracture using LDSC27 and found a significantly positive correlation between them (rg=0.21, SE=0.03, p<0.001) (online supplemental table 7). We then used MiXeR to quantify the polygenic overlap (eg, the number of unique and shared polygenic SNPs) for MDD and fracture28, and also demonstrated a significantly positive correlation (rg=0.23, SE=0.02) (figure 3A and online supplemental table 7). Of the 1069 causal variants linked to fracture, 866 SNPs (SE=0.1) were also shared with MDD, while the overall measure of polygenic overlap, on a 0%–100% scale, was 12.97% (as quantified by the Dice coefficient) (online supplemental table 7). The conditional Q-Q plots, which displayed a consistent increase in leftward deflection for subsets of variants with higher significance in the conditional trait in both directions (MDD given fracture and fracture given MDD), further indicated substantial polygenic overlap between MDD and fracture (figure 3B).
Figure 3.
Genetic overlap between MDD and fracture. (A) The shared number of variants between MDD and fracture. (B) The conditional Q-Q plots at the level of p<0.1, p<0.01, p<0.001. (C) The genetic variants jointly associated with MDD and fracture at conjFDR <0.05. (D) The forest plot of the four shared SNPs to show the association with MDD and fracture. (E) The association of genetically predicted MDD with fracture and vice versa. (F) Visualised the association of lead SNPs for MDD with the risk of fracture. conjFDR, conjunctional false discovery rate; MDD, major depressive disorder; Q-Q, quantile-quantile; SNPs, single nucleotide polymorphisms.
We used the condFDR/conjFDR approach to identify the specific shared loci and SNPs jointly associated with MDD and fracture. Four distinct genomic loci (rs7554101, rs310749, rs10217595 and rs2311117) were identified as jointly associated with MDD and fracture (figure 3C and online supplemental table 8), with the top SNP rs7554101 in the intron of gene SGIP1 (conjFDR=0.008). By comparing the effect directions of the lead SNPs at the shared loci (conjFDR < 0.05), we found that three lead SNPs on chromosomes 1, 9 and 18 had consistent effect directions in MDD and fracture, while one SNP (rs310749) exhibited opposite effects (figure 3D). Further, we calculated the wGRS for the UK Biobank participants with these four SNPs. When we regressed the observed fracture on the wGRS, we found that the genetically predicted MDD was associated with a higher risk of fracture (OR=1.03, 95% CI 1.01 to 1.06, p=0.049) (figure 3E). Also, the genetically predicted fracture was associated with a higher risk of MDD (OR=1.04, 95% CI 1.02 to 1.07, p<0.001) (figure 3E), with stronger effects in women than in men (online supplemental figure 3). Moreover, the Mendelian randomisation-egger regression also suggested a positive association between MDD and fracture (OR=1.10, 95% CI 1.04 to 1.16, p<0.001) (figure 3F). The individual effect of the SNPs for MDD on fracture was adjusted using a false discovery rate of <0.05.36 Among the 74 lead SNPs analysed of MDD, two SNPs (including rs6656912 in the intron of gene SGIP1, whose r2 with the rs7554101 is 0.79) remained as potential variants with effects on fracture (figure 3F and online supplemental table 5).
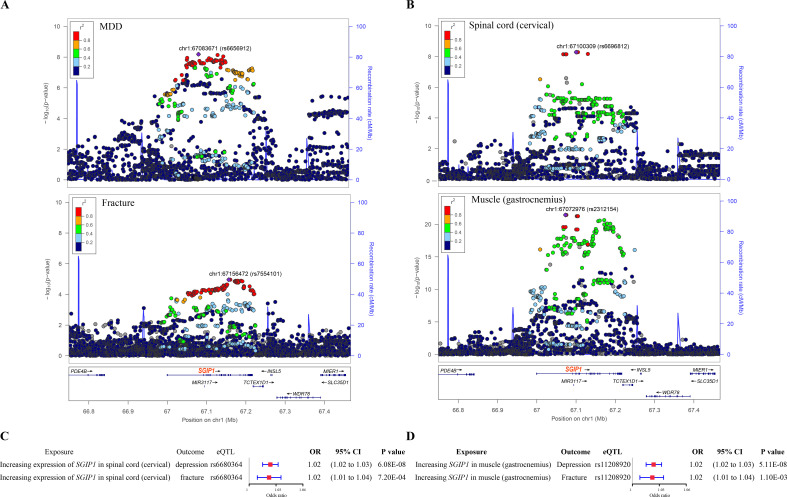
We identified a genomic locus approximately 250 kb upstream and downstream of the gene SGIP1 (hg19, chr1: 66749044–67466822), which harbours a number of SNPs associated with MDD and fracture (figure 4A). This locus was also associated with the expression of SGIP1 in the spinal cord (cervical) and muscle (gastrocnemius) (figure 4B). Further, by applying the SMR method31 with the eQTL summary data of SGIP1 in the two tissues and the genetic summary data of MDD and fracture, we found that higher expression of SGIP1 (ENSG00000118473) in the spinal cord was associated with an increased risk of MDD (OR=1.02, 95% CI 1.02 to 1.03, p<0.001) and an increased risk of fracture (OR=1.02, 95% CI 1.01 to 1.04, p<0.001) (figure 4C and online supplemental table 9). Meanwhile, the higher expression of SGIP1 (ENSG00000118473) in muscle was associated with an increased risk of fracture (OR=1.02, 95% CI 1.01 to 1.03, p=0.001) and an increased risk of MDD (OR=1.02, 95% CI 1.02 to 1.03, p<0.001) (figure 4D and online supplemental table 9).
Figure 4.
The distinct signal (SGIP1) shared by MDD and fracture. (A) The regional plot of the association between MDD and fracture. The x axis is the position at chr1: 66749044–67466822 (hg19). Each dot represents a genetic variant within this region. The upper panel shows the association of genetic variants with MDD, with the most significant SNP rs6656912 shown in purple diamond. The lower panel shows the association of genetic variants with fracture, with the most significant SNP rs7554101 shown in purple diamond. (B) The regional plot of the association of SGIP1 gene expression in the spinal cord (cervical) and muscle (gastrocnemius). The upper panel shows the association of SGIP1 gene expression in the spinal cord (cervical), with the most significant SGIP1 eQTL rs6696812 shown in purple diamond. The lower panel shows the association of SGIP1 gene expression in muscle (gastrocnemius), with the most significant SGIP1 eQTL rs2312154 shown in purple diamond. (C) The association of genetically predicted SGIP1 gene expression with MDD and fracture risk in spinal cord (cervical). (D) The association of genetically predicted SGIP1 gene expression with MDD and fracture risk in muscle (gastrocnemius). eQTL, expression quantitative trait loci; MDD, major depressive disorder; SNP, single nucleotide polymorphism.
Finally, we mapped the four shared genetic variants to 53 genes using two strategies (see Methods) (online supplemental table 10). Enrichment analysis of the 53 genes within the reported genes in the GWAS catalogue37 revealed that these genes were significantly enriched in waist-to-hip ratio, ability to confide in someone, loneliness and heel bone mineral density (online supplemental file 4A). Moreover, within the GTEx tissues, we found that the 53 genes were enriched as differentially expressed gene sets in brain tissues (hypothalamus, hippocampus, amygdala, substantia nigra and cortex) compared with other tissue types (online supplemental figure 4B).
Discussion
Main findings
In this study, using the UK Biobank prospective cohort, we found an association between MDD and the risk of subsequent fracture. With the same cohort but different time series data, we observed a higher risk of MDD following a fracture. To further elucidate this complex relationship, we used the genetic data to evaluate the genetic overlap between MDD and fracture. Our findings suggest significant genetic pleiotropy between MDD and fracture, aligning with the bidirectional association observed in the epidemiological analysis.
Both MDD and osteoporosis/fracture are prevalent conditions with substantial impacts on morbidity, mortality and quality of life. While limited evidence suggests that osteoporosis might exacerbate depressive symptoms, more studies show that depression is associated with decreased bone density and increased fracture risk.18 19 In this study, we used data from the UK Biobank, which is a large-scale epidemiological resource, to investigate the association between MDD and fracture in a prospective context (https://www.ukbiobank.ac.uk/media/gnkeyh2q/study-rationale.pdf). We initially defined the event of fracture first in the UK Biobank data set. Taking the diagnosis date of the fracture as the reference date, we tracked back the cohort and conducted a nested case-control analysis to assess the association between MDD and subsequent fracture. We also prospectively followed the participants from the reference date until the truncation date to assess the association between fracture and subsequent MDD in a matched cohort. Our regression models were adjusted for a variety of confounding factors that may affect both conditions including poor health behaviours (eg, smoking, alcohol drinking, sleeping habit and physical inactivity)38 and socioeconomic status (employment status and education).39 For the model of fracture risk, we further adjusted for falls, BMD and antidepressant medication, as some studies suggested that certain antidepressants, such as selective serotonin reuptake inhibitors, might have adverse effects on bone health.40 In the model of MDD risk, we additionally controlled for pre-existing psychiatric disorders. Our genetic pleiotropic analyses between MDD and fracture suggested a shared component related to the cannabinoid receptor, which prompted us to include cannabis use in an additional model, and the association remained robust. Overall, we observed a bidirectional relationship between MDD and fracture. These underscore the importance for clinicians to develop management plans that address both diseases. For example, Almeida et al suggested that the management of depression in older adults should include strategies for fracture prevention.17 On the other hand, van den Berg et al proposed that clinicians treating patients with recent low-energy fractures should consider not only skeletal-related risk factors for fracture but also fall-related risk factors, including depression.41
The observational findings suggested a longitudinal and bidirectional association between MDD and fracture. We subsequently explored whether common genetic factors might underlie both conditions. We observed a significant genetic correlation between MDD and fracture, with approximately 81% (866 out of 1069) of the genetic variants impacting the risk of fracture also associated with MDD. We calculated the wGRS for the individuals with the SNPs jointly associated with MDD and fracture, and found that the genetically predicted MDD was associated with a higher risk of fracture and vice versa. These results support the genetic evidence for a bidirectional relationship between MDD and fracture. Interestingly, MDD was reported to share substantial genetic liability with other skeleton diseases such as osteoarthritis, suggesting a potential mutual risk between them.42
We identified the gene SGIP1 as a shared genetic component between MDD and fracture. This locus was also reported to be associated with anxiety/stress-related disorders,43 depressive symptoms44 and bone density.12 Interestingly, the protein encoded by the SGIP1 gene inhibits the endocytosis of cannabinoid receptor type 1 (CB1R) and increases its cell surface stability,45 thus modulating CB1R signalling.46 The endocannabinoid system, particularly CB1R, plays a critical role in the regulation of emotional behaviours.47 CB1R is the first cannabinoid identified and mainly expressed in the brain.48 A recent study suggested that the knockdown of CB1R in a neuronal circuit increased synaptic activity and susceptibility to stress/depression.47 CB1 receptors are expressed on nerve fibres within bone49 and bone cells such as osteoblasts, osteoclasts and adipocytes.50 Genetic inactivation of CB1R has been linked to age-related osteoporosis with reduced bone formation and bone marrow adipocyte accumulation.51 Therefore, cannabinoids may have the potential to mitigate arthritis progression, protect against osteoporosis, inhibit the proliferation of bone tumour cells, alleviate bone cancer pain and promote fracture healing.52 Given that genetic deletion of SGIP1 can enhance cannabinoid tetrad behaviour,46 a higher level of SGIP1 may be associated with an increased risk of MDD and fracture, consistent with our results. An illustration of the assumptive mechanism is shown in online supplemental figure 5. The interaction between SGIP1 and CB1R, with functional consequences observed both in vitro and in vivo, implies a potential therapeutic effect for related diseases.53 Research into the SGIP1-CB1R relationship has been primarily motivated by the aim to enhance the therapeutic efficacy of CB1R agonists, including the naturally occurring compound cannabidiol (CBD) extracted from the Cannabis sativa plant. Evidence suggests that synthetic cannabinoids can induce antidepressant-like effects.47 Preliminary clinical trials have underscored the role of CBD in modulating behaviours associated with anxiety and depression, as well as cognition and locomotion.54 55 On the other hand, it was suggested that CBD usage might positively impact bone morphology in an osteoporotic population.56 Both preclinical and clinical studies showed that cannabis-mediated effects through the endocannabinoid system may be a potentially effective treatment option for individuals with osteoporosis.57 Therefore, these findings suggest that modulating the endocannabinoid system could be a potential therapeutic strategy for both MDD and bone loss.
Limitations
This study has some limitations. First, independent replication for the longitudinal phenotypic association between MDD and fracture was not pursued. However, genetic analysis revealed that genetically predicted MDD was associated with a higher risk of fracture, and vice versa. Therefore, the genetic findings, independent of confounding factors, confirmed the bidirectional association in the observational study. Second, although we had a comprehensive discussion on the shared genetic loci (eg, the SGIP1 gene), functional studies are needed to elucidate the genetic overlap between MDD and fracture.
Implications
In summary, our findings revealed both phenotypic and genetic correlations between MDD and fracture. The genetic contribution to both traits, exemplified by the shared loci (such as SGIP1), suggests a common underlying biology. This underscores the potential of modulating the endocannabinoid system as a therapeutic strategy for both diseases.
Acknowledgments
We acknowledge the High-performance Computing Center at Westlake University in China.
Biographies
Pianpian Zhao is currently a postdoctoral fellow in the Laboratory of Disease and Population at Westlake University in Hangzhou, China. She obtained her PhD in biology from Zhejiang University, China in 2023. Dr. Zhao has published papers as a first author in reputable journals including Science Translational Medicine, eLife and the Journal of Epidemiology and Community Health. Additionally, she was involved in several National Natural Science Foundation projects. Her main research interests include the genetics and genomics of complex diseases.

Zhimin Ying is an attending orthopaedic surgeon at The Second Affiliated Hospital, School of Medicine, Zhejiang University, China. He received his PhD degree from the School of Medicine of Zhejiang University. During his PhD study, he also completed a joint PhD program at Harvard Medical School. Dr.Ying has published over ten original articles in journals such as Journal of American College of Surgeons, Experimental Gerontology and Front Nutrition. Dr. Ying’s research interests mainly focus on skeleton aging.

Chengda Yuan graduated from Anhui Medical University in 2007 with a master's degree in Dermatology. He is now the director and chief physician at the Department of Dermatology, Hangzhou Traditional Chinese Medicine Hospital. He is also an associate professor at Zhejiang University of Traditional Chinese Medicine. His current main research direction is related to the susceptibility genes and phenotypes of hereditary diseases.

Footnotes
Contributors: HZheng conceptualised and designed the study. PZ conducted the analyses and drafted the paper. ZY, CY and HZhang helped in clinical interpretation of the results and drafted the manuscript. AD, JT, XY, MY, WJ, WT, GT and DK helped in the data acquisition and manuscript editing. The authors read and approved the final manuscript. The first author, PZ, and the corresponding author, HZheng, are responsible for the overall content as guarantors.
Funding: This study was supported by the ‘Pioneer’ and ‘Leading Goose’ R&D Program of Zhejiang (#2023C03164 and #2024SSYS0032), the National Natural Science Foundation of China (#82370887), the Chinese National Key Technology R&D Program, Ministry of Science and Technology (#2021YFC2501702) and the funds from the Westlake Laboratory of Life Sciences and Biomedicine (#202208014). The funder had no role in study design, data collection, data analysis, data interpretation or writing of this article.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
All individuals provided written informed consent. The North West Multi-Centre Research Ethics Committee approved the UK Biobank ethical application (reference number: 16/NW/0274). Participants gave informed consent to participate in the study before taking part.
References
- 1. Liu Q, He H, Yang J, et al. Changes in the global burden of depression from 1990 to 2017: findings from the global burden of disease study. J Psychiatr Res 2020;126:134–40. 10.1016/j.jpsychires.2019.08.002 [DOI] [PubMed] [Google Scholar]
- 2. Hasin DS, Sarvet AL, Meyers JL, et al. Epidemiology of adult DSM-5 major depressive disorder and its specifiers in the United States. JAMA Psychiatry 2018;75:336–46. 10.1001/jamapsychiatry.2017.4602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yaffe K, Blackwell T, Gore R, et al. Depressive symptoms and cognitive decline in nondemented elderly women: a prospective study. Arch Gen Psychiatry 1999;56:425–30. 10.1001/archpsyc.56.5.425 [DOI] [PubMed] [Google Scholar]
- 4. Devanand DP, Sano M, Tang MX, et al. Depressed mood and the incidence of Alzheimer’s disease in the elderly living in the community. Arch Gen Psychiatry 1996;53:175–82. 10.1001/archpsyc.1996.01830020093011 [DOI] [PubMed] [Google Scholar]
- 5. Luppino FS, de Wit LM, Bouvy PF, et al. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry 2010;67:220–9. 10.1001/archgenpsychiatry.2010.2 [DOI] [PubMed] [Google Scholar]
- 6. Golden SH, Lazo M, Carnethon M, et al. Examining a bidirectional association between depressive symptoms and diabetes. JAMA 2008;299:2751–9. 10.1001/jama.299.23.2751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cizza G, Primma S, Coyle M, et al. Depression and osteoporosis: a research synthesis with meta-analysis. Horm Metab Res 2010;42:467–82. 10.1055/s-0030-1252020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Compston JE, McClung MR, Leslie WD. Osteoporosis. Lancet 2019;393:364–76. 10.1016/S0140-6736(18)32112-3 [DOI] [PubMed] [Google Scholar]
- 9. Ormel J, Hartman CA, Snieder H. The genetics of depression: successful genome-wide association studies introduce new challenges. Transl Psychiatry 2019;9:114. 10.1038/s41398-019-0450-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wray NR, Ripke S, Mattheisen M, et al. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat Genet 2018;50:668–81. 10.1038/s41588-018-0090-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry 2000;157:1552–62. 10.1176/appi.ajp.157.10.1552 [DOI] [PubMed] [Google Scholar]
- 12. Morris JA, Kemp JP, Youlten SE, et al. An atlas of genetic influences on osteoporosis in humans and mice. Nat Genet 2019;51:258–66. 10.1038/s41588-018-0302-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Andrew T, Antioniades L, Scurrah KJ, et al. Risk of wrist fracture in women is heritable and is influenced by genes that are largely independent of those influencing BMD. J Bone Miner Res 2005;20:67–74. 10.1359/JBMR.041015 [DOI] [PubMed] [Google Scholar]
- 14. Wagner H, Melhus H, Pedersen NL, et al. Heritable and environmental factors in the causation of clinical vertebral fractures. Calcif Tissue Int 2012;90:458–64. 10.1007/s00223-012-9592-7 [DOI] [PubMed] [Google Scholar]
- 15. Wu Q, Liu B, Tonmoy S. Depression and risk of fracture and bone loss: an updated meta-analysis of prospective studies. Osteoporos Int 2018;29:1303–12. 10.1007/s00198-018-4420-1 [DOI] [PubMed] [Google Scholar]
- 16. Yuan S, Chen J, Zeng L, et al. Association of bone mineral density and depression in different bone sites and ages: a meta-analysis. Food Sci Nutr 2021;9:4780–92. 10.1002/fsn3.2379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Almeida OP, Hankey GJ, Golledge J, et al. Depression and the risk of fractures in later life: the health in men cohort study. Maturitas 2021;145:6–11. 10.1016/j.maturitas.2020.12.004 [DOI] [PubMed] [Google Scholar]
- 18. Aloumanis K, Mavroudis K. “The "depressive" face of osteoporosis and the "osteoporotic" face of depression”. Hormones (Athens) 2013;12:350–62. 10.1007/BF03401301 [DOI] [PubMed] [Google Scholar]
- 19. Carlone C, Rusconi AC, Valeriani G, et al. Osteoporosis and major depression: open debate on a bidirectional relationship. Riv Psichiatr 2015;50:161–7. 10.1708/2002.21642 [DOI] [PubMed] [Google Scholar]
- 20. Williams LJ, Berk M, Henry MJ, et al. Depression following fracture in women: a study of age-matched cohorts. BMJ Open 2014;4:e004226. 10.1136/bmjopen-2013-004226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. He B, Lyu Q, Yin L, et al. Depression and osteoporosis: a Mendelian randomization study. Calcif Tissue Int 2021;109:675–84. 10.1007/s00223-021-00886-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xia J, Xie S-Y, Liu K-Q, et al. Systemic evaluation of the relationship between psoriasis, psoriatic arthritis and osteoporosis: observational and Mendelian randomisation study. Ann Rheum Dis 2020;79:1460–7. 10.1136/annrheumdis-2020-217892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xia J-W, Zhang L, Li J, et al. Both indirect maternal and direct fetal genetic effects reflect the observational relationship between higher birth weight and lower adult bone mass. BMC Med 2022;20:361. 10.1186/s12916-022-02531-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhu X-W, Liu K-Q, Yuan C-D, et al. General and abdominal obesity operate differently as influencing factors of fracture risk in old adults. iScience 2022;25:104466. 10.1016/j.isci.2022.104466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ernster VL. Nested case-control studies. Prev Med 1994;23:587–90. 10.1006/pmed.1994.1093 [DOI] [PubMed] [Google Scholar]
- 26. Wegman Me. International classification of diseases. Pediatrics 1959;23:761–5. [PubMed] [Google Scholar]
- 27. Bulik-Sullivan BK, Loh P-R, Finucane HK, et al. LD score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat Genet 2015;47:291–5. 10.1038/ng.3211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Frei O, Holland D, Smeland OB, et al. Bivariate causal mixture model quantifies polygenic overlap between complex traits beyond genetic correlation. Nat Commun 2019;10:2417. 10.1038/s41467-019-10310-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schwartzman A, Lin X. The effect of correlation in false discovery rate estimation. Biometrika 2011;98:199–214. 10.1093/biomet/asq075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Andreassen OA, Thompson WK, Schork AJ, et al. Improved detection of common variants associated with schizophrenia and bipolar disorder using pleiotropy-informed conditional false discovery rate. PLoS Genet 2013;9:e1003455. 10.1371/journal.pgen.1003455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhu Z, Zhang F, Hu H, et al. Integration of summary data from GWAS and eQTL studies predicts complex trait gene targets. Nat Genet 2016;48:481–7. 10.1038/ng.3538 [DOI] [PubMed] [Google Scholar]
- 32. GTEx Consortium . Genetic effects on gene expression across human tissues. Nature 2017;550:204–13. 10.1038/nature24277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Võsa U, et al. Unraveling the polygenic architecture of complex traits using blood eQTL metaanalysis. 2018. 10.1101/447367 [DOI]
- 34. Watanabe K, Taskesen E, van Bochoven A, et al. Functional mapping and annotation of genetic associations with FUMA. Nat Commun 2017;8:1826. 10.1038/s41467-017-01261-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Welter D, MacArthur J, Morales J, et al. The NHGRI GWAS Catalog, a curated resource of SNP-trait associations. Nucleic Acids Res 2014;42:D1001–6. 10.1093/nar/gkt1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Benjamini Y, Hochberg Y. Controlling the false discovery rate - a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B (Methodological) 1995;57:289–300. 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- 37. Buniello A, MacArthur JAL, Cerezo M, et al. The NHGRI-EBI GWAS Catalog of published genome-wide association studies, targeted arrays and summary statistics 2019. Nucleic Acids Res 2019;47:D1005–12. 10.1093/nar/gky1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mezuk B, Eaton WW, Golden SH. Depression and osteoporosis: epidemiology and potential mediating pathways. Osteoporos Int 2008;19:1–12. 10.1007/s00198-007-0449-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Freeman A, Tyrovolas S, Koyanagi A, et al. The role of socio-economic status in depression: results from the COURAGE (aging survey in Europe). BMC Public Health 2016;16:1098. 10.1186/s12889-016-3638-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Prieto-Alhambra D, Petri H, Goldenberg JSB, et al. Excess risk of hip fractures attributable to the use of antidepressants in five European countries and the USA. Osteoporos Int 2014;25:847–55. 10.1007/s00198-013-2612-2 [DOI] [PubMed] [Google Scholar]
- 41. van den Berg M, Verdijk NA, Leusink GL, et al. Depression after low-energy fracture in older women predicts future falls: a prospective observational study. BMC Geriatr 2011;11:73. 10.1186/1471-2318-11-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang F, Rao S, Baranova A. Shared genetic liability between major depressive disorder and osteoarthritis. Bone Joint Res 2022;11:12–22. 10.1302/2046-3758.111.BJR-2021-0277.R1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mei L, Gao Y, Chen M, et al. Overlapping common genetic architecture between major depressive disorders and anxiety and stress-related disorders. Prog Neuropsychopharmacol Biol Psychiatry 2022;113:110450. 10.1016/j.pnpbp.2021.110450 [DOI] [PubMed] [Google Scholar]
- 44. Howard DM, Adams MJ, Clarke T-K, et al. Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat Neurosci 2019;22:343–52. 10.1038/s41593-018-0326-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hájková A, Techlovská Š, Dvořáková M, et al. SGIP1 alters internalization and modulates signaling of activated cannabinoid receptor 1 in a biased manner. Neuropharmacology 2016;107:201–14. 10.1016/j.neuropharm.2016.03.008 [DOI] [PubMed] [Google Scholar]
- 46. Dvorakova M, Kubik-Zahorodna A, Straiker A, et al. SGIP1 is involved in regulation of emotionality, mood, and nociception and modulates in vivo signalling of cannabinoid CB(1) receptors. Br J Pharmacol 2021;178:1588–604. 10.1111/bph.15383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shen C-J, Zheng D, Li K-X, et al. Cannabinoid CB(1) receptors in the amygdalar cholecystokinin glutamatergic afferents to nucleus accumbens modulate depressive-like behavior. Nat Med 2019;25:337–49. 10.1038/s41591-018-0299-9 [DOI] [PubMed] [Google Scholar]
- 48. Matsuda LA, Lolait SJ, Brownstein MJ, et al. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 1990;346:561–4. 10.1038/346561a0 [DOI] [PubMed] [Google Scholar]
- 49. Tam J, Ofek O, Fride E, et al. Involvement of neuronal cannabinoid receptor Cb1 in regulation of bone mass and bone remodeling. Mol Pharmacol 2006;70:786–92. 10.1124/mol.106.026435 [DOI] [PubMed] [Google Scholar]
- 50. Saponaro F, Ferrisi R, Gado F, et al. The role of cannabinoids in bone metabolism: a new perspective for bone disorders. Int J Mol Sci 2021;22:12374. 10.3390/ijms222212374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Idris AI, Sophocleous A, Landao-Bassonga E, et al. Cannabinoid receptor type 1 protects against age-related osteoporosis by regulating osteoblast and adipocyte differentiation in marrow stromal cells. Cell Metab 2009;10:139–47. 10.1016/j.cmet.2009.07.006 [DOI] [PubMed] [Google Scholar]
- 52. Xin Y, Tang A, Pan S, et al. Components of the endocannabinoid system and effects of cannabinoids against bone diseases: a mini-review. Front Pharmacol 2021;12:793750. 10.3389/fphar.2021.793750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Durydivka O, Mackie K, Blahos J. SGIP1 in axons prevents internalization of desensitized CB1R and modifies its function. Front Neurosci 2023;17:1213094. 10.3389/fnins.2023.1213094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. García-Gutiérrez MS, Navarrete F, Gasparyan A, et al. Cannabidiol: a potential new alternative for the treatment of anxiety, depression, and psychotic disorders. Biomolecules 2020;10:1575. 10.3390/biom10111575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. de Mello Schier AR, de Oliveira Ribeiro NP, Coutinho DS, et al. Antidepressant-like and anxiolytic-like effects of cannabidiol: a chemical compound of Cannabis sativa. CNS Neurol Disord Drug Targets 2014;13:953–60. 10.2174/1871527313666140612114838 [DOI] [PubMed] [Google Scholar]
- 56. Ihejirika-Lomedico R, Patel K, Buchalter DB, et al. Non-psychoactive cannabidiol prevents osteoporosis in an animal model and increases cell viability, proliferation, and osteogenic gene expression in human skeletal stem and progenitor cells. Calcif Tissue Int 2023;112:716–26. 10.1007/s00223-023-01083-2 [DOI] [PubMed] [Google Scholar]
- 57. Clouse G, Penman S, Hadjiargyrou M, et al. Examining the role of cannabinoids on osteoporosis: a review. Arch Osteoporos 2022;17:146. 10.1007/s11657-022-01190-x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
gpsych-2023-101418supp001.pdf (792.8KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.