Abstract
Introduction
The use of androgenic anabolic steroids (AASs) among recreational athletes is steadily increasing. However, knowledge regarding the potentially harmful effects of AAS primarily originates from case reports and small observational studies. This large-scale study aims to investigate the impact of AAS use on vascular plaque formation, preclinical coronary disease, cardiac function, circulating cardiovascular risk markers, quality of life (QoL) and mental health in a broad population of illicit AAS users.
Methods and analyses
A nationwide cross-sectional cohort study including a diverse population of men and women aged ≥18 years, with current or previous illicit AAS use for at least 3 months. Conducted at Odense University Hospital, Denmark, the study comprises two parts. In part A (the pilot study), 120 recreational athletes with an AAS history will be compared with a sex-matched and age-matched control population of 60 recreational athletes with no previous AAS use. Cardiovascular outcomes include examination of non-calcified coronary plaque volume and calcium score using coronary CT angiography, myocardial structure and function via echocardiography, and assessing carotid and femoral artery plaques using ultrasonography. Retinal microvascular status is evaluated through fundus photography. Cardiovascular risk markers are measured in blood. Mental health outcomes include health-related QoL, interpersonal difficulties, body image concerns, aggression dimensions, anxiety symptoms, depressive severity and cognitive function assessed through validated questionnaires. The findings of our comprehensive study will be used to compose a less intensive investigatory cohort study of cardiovascular and mental health (part B) involving a larger group of recreational athletes with a history of illicit AAS use.
Ethics and dissemination
The study received approval from the Regional Committee on Health Research Ethics for Southern Denmark (S-20210078) and the Danish Data Protection Agency (21/28259). All participants will provide signed informed consent. Research outcomes will be disseminated through peer-reviewed journals and scientific conferences.
Trial registration number
Keywords: Cardiovascular imaging, General endocrinology, Cardiology, Cardiomyopathy, Sex steroids & HRT
STRENGTHS AND LIMITATIONS OF THIS STUDY.
The current research project evaluates cardiovascular health in both male and female recreational athletes with a self-reported illicit androgenic anabolic steroids (AASs) intake of at least 3 months.
The study will offer participants an extensive health examination and provide important descriptive data regarding AAS history, as well as information on self-reported somatic and psychological health status through questionnaires.
Our findings may help to develop optimal investigatory screening programmes for a larger cohort study of recreational athletes with a history of illicit AAS use (part B).
We aim to include a broad spectrum of recreational athletes with a history of AAS use, but our study participants may not be representative of all recreational athletes using AAS.
Some of our data are subject to bias, including the self-reported information on AAS use, such as type and length of steroid intake, dosage and the administration forms. Furthermore, training routines and the intake of so-called recreational drugs may vary.
Introduction
Androgenic anabolic steroid (AAS) is the common denominator of testosterone and its synthetic derivates, and this group of drugs has received growing awareness because of its widespread and increasing use among recreational athletes.1 Thus, a Danish report from 2010 estimated the prevalence of AAS using recreational athletes to 44 000, equivalent to 0.8% of the total Danish population. This estimate corresponds well with reports from other countries.2 Although there is a clear sex difference in the prevalence of illicit AAS use, with men constituting the majority of users, it is estimated that an escalating number of women are also using these substances.1 3 However, specific health data concerning these women remain unavailable.
The illicit use of AAS may develop into an overt drug dependence. Thus, it has been reported that 30%–40% of AAS using subjects after cessation have to resume their illicit intake of AAS due to severe withdrawal symptoms, thereby developing a chronic AAS dependence syndrome.4 This long-term exposure to AAS may lead to serious adverse health effects that often appear in middle age.4 5
Indeed, the mortality among recreational athletes using AAS is 6–20 fold higher than that of athletes not using AAS, and almost one-third of the deaths can be attributed to cardiovascular disease.6 In accordance with this, a recent Danish register study reported that the mortality and incidence of non-ischaemic heart disease were three times higher in AAS users compared with the background population.7 These findings are worrying and indicate that the adverse health effects of AAS remain greatly underappreciated.4
Originally, the association between AAS and myocardial infarction was based on case reports and small observational studies of male AAS users, but within the last decades, studies that substantiate the association have emerged.6 8–13 In 2006, a cross-sectional study including 14 AAS using male bodybuilders with a mean age of 39 years showed that 50% of the participants had coronary atherosclerosis and a significantly increased coronary calcium score (Agatston score).14 The expected number in this age group would have been 3 according to population studies.15 Similar findings were made in a coronary CT angiography (CCTA) study of young male subjects (23–43 years), where 24% of the AAS users had significant coronary atherosclerosis compared with none among non-AAS-using subjects.16 In one of the largest studies available, comprising 86 male weightlifters (34–54 years) with ≥2 years of cumulative lifetime AAS use and 54 non-ASS using weightlifters, AAS users demonstrated higher coronary artery plaque volume than non-users, being associated with the cumulative lifetime duration of AAS intake.17 Lastly, a CCTA-based study conducted in 2021 in Norway showed that coronary atherosclerosis was present in seven out of 41 male AAS users (17%) with a mean age of 33 years.18 Thus, there is ample evidence that an intake of AAS is associated with premature atherosclerosis. As opposed to the coronary arteries, there are virtually no data describing carotid and femoral vascular plaque formation in male or female recreational athletes with an illicit AAS user. Ultrasound of carotid and femoral vessels may be useful for identifying subjects at risk of cardiovascular events,19 20 and therefore, we believe it is worth examining whether ultrasound can provide useful information regarding macrovascular disease in both male and female AAS users. Interestingly, two small case studies demonstrated a possible association between AAS use and retinal vascular occlusion21 22 in male AAS using bodybuilders, indicating that AAS use may also involve smaller vessels. Finally, there are no available data on atherosclerotic disease in female AAS users.
In addition to proatherosclerotic effects, there is comprehensive evidence demonstrating that illicit AAS use induces myocardial changes, leading to impaired systolic and diastolic function17 23–28 in males. Autopsies29–31 and studies using echocardiography25–28 32 33 and cardiac MRI23 24 have identified a potential AAS-related cardiomyopathy characterised by left ventricular (LV) remodelling or hypertrophy with increased relative wall thickness and/or total ventricular mass,17 18 24 26 33 34 a potential increase of heart chamber dimensions,17 24 evident changes of ventricular relaxation and diastolic function,17 27 33 a reduced LV systolic function17 18 24 26 33 34 and possibly a greater prevalence of cardiac fibrosis.35 36
Finally, there appears to be an association between long-term illicit AAS and mental health problems.4 37 Studies report that both male and female AAS users show symptoms of muscle dysmorphia,38 anxiety and depression,3 39 and lower self-esteem.3 38 In addition, an illicit AAS use has been linked to an increased prevalence of psychopathic traits,39 40 sexual and substance use risk-taking behaviours3 38 and anger problems,40 when compared with non-users.41 42 Unfortunately, AAS discontinuation does not necessarily lead to a fast recovery.4 Many illicit AAS users—especially males—complain about severe fatigue and depression after discontinuation, and the weakened mental health may persist for as long as 2–3 years after AAS discontinuation, and in some cases becomes permanent.5 Accumulating evidence suggests that the biological effects of AASs on emotional and cognitive brain regions may contribute to violent and criminal behaviours, but there is still limited knowledge regarding premorbid psychopathology in AAS users.42 Our data obtained through validated questionnaires will deliver important information on mental health status in individuals with short and long-term AAS abuse.
As most data originate from selected groups of strength athletes and weightlifters, it remains to be elucidated whether findings are representative of the general population of recreational athletes illicitly using AAS. Moreover, no cardiovascular data on female AAS users exist. Therefore, we found it timely to conduct a large-scale study with the aim to increase knowledge regarding the cardiovascular and mental health status in the broad population of both male and female recreational athletes and to develop a relevant screening programme which could possibly be employed in other AAS-using populations.
Aim
The aim of our study is to investigate the associations between AAS use and various health outcomes within an expanding population of illicit AAS users, encompassing not only strength trainers and weightlifters, but also a variety of recreational athletes engaged in different forms of physical activity. This population encompasses individuals with different fitness objectives and motivations, varying demographic profiles and distinct social backgrounds, who have engaged in an illicit AAS use for a minimum of 3 months. Our hypothesis posits that the dose and duration of AAS use are linked to an elevated risk of preclinical cardiovascular diseases, including the development of carotid and femoral plaques, preclinical coronary disease, myocardial dysfunction and alterations in retinal microvasculature. Additionally, we hypothesise that AAS users will exhibit lower health-related quality of life (QoL), increased interpersonal difficulties, elevated body image concerns, aggression tendencies and a higher prevalence of anxiety, depression and cognitive alterations compared with a control population.
Methods and analysis
Study population and recruitment
Our primary focus is to thoroughly investigate a diverse and heterogeneous population of recreational athletes engaging in AAS use, considering a range of motivations that may include various AAS use patterns, training objectives, exercise regimens and differences in mental well-being among participants. The inclusivity of this study is underscored by the deliberate decision to establish a minimum requirement of 3 months of illicit AAS use, without specifying a particular minimum weekly androgen dose.
Before recruitment, we sought the input of three long-term AAS users on research relevance, recruitment strategies and outcomes, incorporating their insights into the ethical committee application. The application was subsequently reviewed and approved by the local ethical committee (S-2021007).
To promote the project, we engaged notable recreational athletes with significant digital influence on platforms such as Facebook and Instagram. Our recruitment efforts involve targeted announcements, social media posts, flyers, advertisements and posters in training centres. Potential interested participants receive detailed information through email and telephone correspondence. All participants are enrolled following informed written and oral consent.
We will then meticulously pair AAS users with a healthy group of recreational athletes without any history of AAS use, ensuring individuals have similar sex and age demographics. To ensure the control group accurately reflects a healthy general population, we have set an inclusion criterion for control subjects to engage in strength training at a minimum frequency of twice a week. Control subjects are recruited through similar methods as AAS users, using posters, flyers and social media announcements. We are not performing hair analyses, but we indirectly confirm the current non-use through the measurement of FSH, LH and testosterone levels. In addition, we will add measurements of androgen-related substances in the urine to validate our cohort.
Study design
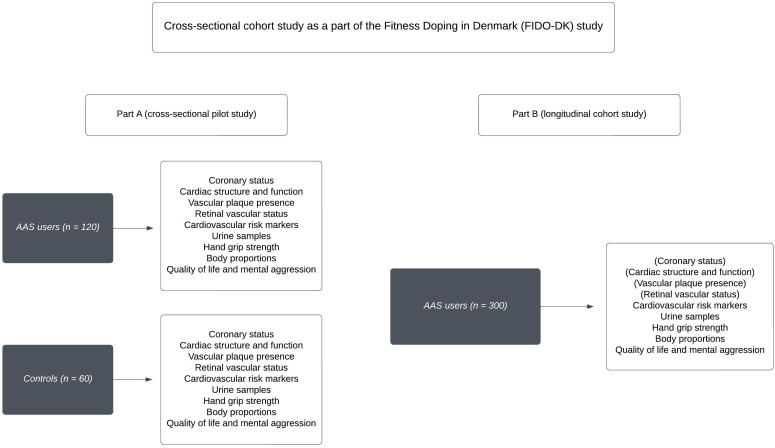
We will conduct a nationwide cross-sectional cohort study originating from Odense University Hospital, Denmark, with the primary objective of establishing the groundwork for a larger prospective observational cohort study, the Fitness Doping in Denmark study (part B). Study part A includes 120 current and former AAS users, and 60 physically active strength training control subjects matched for sex and age. Due to uncertainties regarding the age, sex and duration of AAS use among the participants, the research project begins with an exploratory pilot study. Additionally, we aim to determine the detectability of plaque in the targeted population to assess the feasibility of conducting specific plaque examinations. It enables us to refine and adjust our research questions and methods, including cardiovascular examinations and questionnaires, based on early findings. This approach ensures that our methods are precisely tailored to capture various health outcomes within a diverse population of AAS users. Additionally, this phase facilitates the collection of crucial data for power calculations, helps assess the feasibility of recruiting a sufficient number of participants and evaluates the financial, technical, administrative and logistic aspects of conducting the full-scale study (part B).
After the initial screening process via email and telephone calls, eligible participants will be invited to partake in a comprehensive 3-hour examination programme (part A) at the outpatient clinic of the Department of Cardiology at Odense University Hospital.
On arrival, we will conduct screening of urine, and blood samples for testosterone, follicle-stimulating hormone (FSH), luteinizing hormone (LH) and oestradiol. After having finalised the pilot study, urine samples will be dispatched to a World Anti-Doping Agency (WADA)-accredited laboratory for the analysis of steroid metabolites (Cologne, Germany). Subsequently, participants will be interviewed regarding their medical history, encompassing chronic diseases, allergies, prescribed medication usage, supplement intake, alcohol and tobacco consumption, drug use, dietary habits, physical activity routines and socioeconomic status.
Following this, participants will be required to provide a detailed and structured account of their fitness training history. We will incorporate an assessment of all participants’ weekly exercise intensity and volume using questionnaires. This will allow us to gather detailed information on both strength training and endurance training patterns for both illicit AAS users and controls. In addition, we have added information on their personal record as regards bench press (in kilogram), as this measure is often used as a strength indicator. To obtain a present objective measurement of strength, we also determine handgrip strength (HGS) as quantified using the Jamar Hand Dynamometer.
As regards experiences with AAS, we aim to collect details on current or past AAS use, intervals between intake, types of AAS, dosage, duration of use, age of onset of AAS use, maximum weekly dose of AAS and cumulative lifetime use of AAS. Participants also be queried about the possible illicit intake of other performance-enhancing drugs, such as human growth hormone and selective oestrogen receptor modulators, as well as recreational drugs like cocaine, hash, ecstasy, gamma-hydroxybutyrate (fantasy), heroin, lysergic acid diethylamide (LSD) or any others. This information will be collected through structured questionnaires. Furthermore, participants undergo inquiries related to various psychometric measures, encompassing the assessment of psychiatric diseases, health-related QoL, mental aggression, self-perception and cognitive function. Subsequently, measurements of height, weight, waist and hip circumferences, and calculation of body mass index (BMI, kg/m²) will be recorded. Body composition will be determined using bioelectrical impedance spectroscopy apparatus (SOZO).
The pilot study (part A) includes a comprehensive cardiac investigation programme featuring cardiac CT scans with and without contrast, along with echocardiography. This decision is based on the well-documented association between illicit AAS use and coronary atherosclerosis.16–18 The primary endpoints are the presence of femoral and coronary plaques as estimated by ultrasound, whereas secondary endpoints include coronary artery status as determined by cardiac CT scan with contrast (ie, NCP volume) and without contrast (Agatston score).
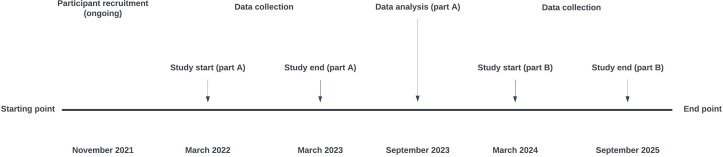
A subsequent larger study (part B) includes participants, who will undergo a targeted examination programme with the most sensitive and applicable cardiovascular examinations (selected among those in the pilot study, that is, CCTA with or without contrast, echocardiography, ultrasonography or retinal fundus photo), together with circulating cardiovascular risk markers, body composition and questionnaires assessing mental status and QoL (figure 1). In addition, we collect urine samples and serum/plasma for biobanking (table 1). Illicit AAS users meet the same eligibility criteria as for study part A, and inclusion of AAS users started as soon as data from study part A were evaluated by the authors. Participant recruitment commenced in November 2021. Data collection for part A has been concluded in March 2023. Data analysis and evaluation were finalised in the autumn of 2023, and a new programme for part B was established. The commencement of part B is anticipated in Q1, 2024, with the entire study projected to conclude by the end of 2025 (figure 2).
Figure 1.
Study design. AAS, androgenic anabolic steroid.
Table 1.
Study outcomes
| Investigations | Pilot study (part A) |
Subsequent cohort study (part B) |
| Non-contrast coronary CT | X | (X) |
| Coronary CT angiography | X | (X) |
| Echocardiography | X | (X) |
| Ultrasound of the carotid and femoral arteries | X | (X) |
| Retinal fundus photo | X | (X) |
| Grip strength measurement | X | X |
| Bioelectrical impedance | X | X |
| Blood samples—immediate analyses+later analyses | X | X |
| Clinical examination | X | X |
| Medical history and socioeconomic status | X | X |
| Questionnaires | X | X |
Our study aims to explore cardiovascular and mental health in Danish recreational athletes with a history of AAS use. Part A involves 120 AAS users and 60 non-users for comparison. Part B, a larger cohort study, will be based on part A findings, with examinations focused on those providing the most value. The inclusion of certain examinations in part B depends on part A results.
AAS, androgenic anabolic steroid.
Figure 2.
Study time line.
Patient and public involvement
Before writing the protocol, we discussed the project and outcome parameters with three long-term AAS users, who provided feedback on the project before its submission to the local ethics committee. All enrolled participants will receive detailed information about their participation. Individuals within the AAS user community who have significant digital platforms (eg, Facebook and Instagram) have been contacted and given comprehensive information about the project. Some of these individuals have also contributed insights regarding the research’s relevance, recruitment strategies, expected outcomes and have assisted in promoting the project.
Endpoints
The initial pilot study (part a) will contain the following endpoints.
Primary endpoint
Carotid and femoral artery plaque development as determined by ultrasound.
Secondary endpoints
Calcium score (Agatston score) as determined by CCTA without contrast.
Non-calcified plaque volume (NCPV) assessed via CCTA with contrast.
Myocardial structure and function evaluated through echocardiography.
Retinal microvascular alterations.
Hand grip strength.
Body composition.
Serum levels of sex hormones and steroids.
Circulating cardiovascular risk and inflammation markers.
Questionnaires including somatic and psychological health status and QoL.
Additionally, body composition, serum levels of sex hormones and steroids, circulating cardiovascular risk markers and questionnaires will account for the secondary endpoints (figure 1). We will conduct separate analyses for men and women, acknowledging the observed variations in AAS doses between sexes. This strategic approach is undertaken to explore and ascertain whether men and women experience similar risks and consequences associated with AAS use, providing a nuanced understanding of potential sex-specific effects. In the later stages of the analyses, we will also conduct a detailed examination of various subgroups within the population. This will include an investigation into long-term users, exploring correlations between extended AAS use and various endpoints. Additionally, subsequent sensitivity analyses will encompass risk factors such as age, BMI, family history of coronary disease, smoking status, substance abuse and levels of physical activity.
Outcomes
An overview of outcomes distributed between the two study parts is outlined in table 1.
All investigations as a part of the pilot study (part A) are performed during one single visit at Odense University Hospital, Denmark.
Coronary CT angiography
CCTA (using Siemens Force CT scanner) is conducted during the visit at Odense University Hospital to examine the presence of coronary atherosclerotic plaques (figure 3). Both NCPV and calcified plaques may be detected by the angiography43 in combination with a semiautomatic computer programme.44 Scanning protocol depends on the heart rate. In patients with a stable heart rate above 60 beats per minute (BPM), intravenously β-blocker is administered until the heart rate is appropriate (if possible below 60 BPM), and a prospectively gated end-diastolic scan is used. In patients with a heart rate >70 BPM despite β-blocker pretreatment or in case of an irregular heart rhythm, a prospective scan 250–400 ms after the QRS complex is performed. Additionally, sublingual nitrates are administered prior to the scan. Drugs such as β-blockers and nitrates are administrated in accordance with daily clinical practice. Experienced cardiologists perform data analyses of NCPs, calcium score/stenosis and pericardial fat using a semiautomatic programme (figure 4).
Figure 3.
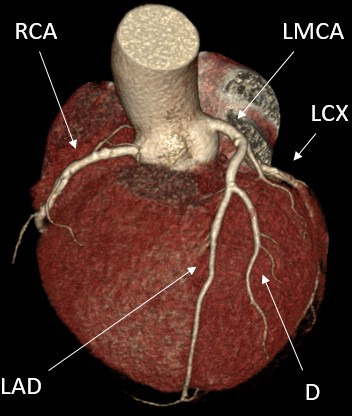
The figure displays a coronary CT angiography. A semiautomatic programme detects non-calcified coronary plaque. CX, circumflex artery; D, diagonal branch of LAD; LAD, left anterior descending artery; LMCA, left main coronary artery; RCA, right coronary artery.
Figure 4.
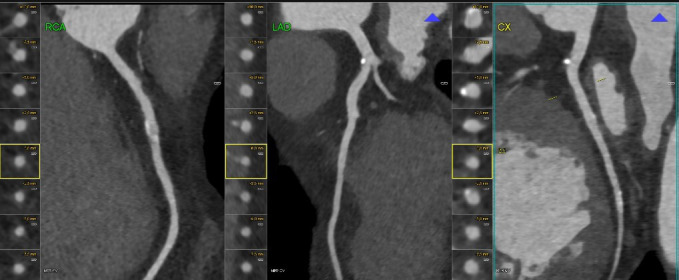
A semiautomatic programme detects non-calcified coronary plaque. CX, circumflex artery; LAD, left anterior descending artery; RCA, right coronary artery.
Echocardiography
A comprehensive transthoracic echocardiography (GE Vivid E95) is performed by a medical doctor. The recordings are stored digitally for blinded analysis. The following are included, namely: size and dimensions of left ventricle, LV and right ventricular (RV) systolic function, LV diastolic function and heart valve function. LV and RV systolic function are measured using global longitudinal strain (GLS) analyses, and Simpson’s biplane method of disks is used specifically for assessing left ventricular ejection fraction (LVEF). Diastolic function is assessed by peak mitral inflow velocity (E), early LV relaxation velocity E’ (septal and lateral E’ values) and left atrial volume.
Ultrasound of carotid and femoral arteries
A non-invasive and painless diagnostic test, known as carotid and femoral artery ultrasound, employs high-frequency ultrasound waves to produce detailed images of the carotid and femoral arteries. This imaging technique enables the estimation of various vascular parameters, including the diameter of the arteries, the thickness of the intima–media layers and the identification of plaques and calcifications.45 By using ultrasound technology, this examination provides valuable insights into the structural integrity and health of the arterial walls, aiding in the assessment of potential vascular abnormalities and informing clinical decisions regarding cardiovascular health.
Retinal fundus photo
A specialised non-invasive fundus camera (Topcon TRC-50DX) consisting of an intricate microscope is used to take two retinal photos of the non-dominant eye after dilatation of the pupil. One photo focusing on macula and another photo focusing on the optic nerve are assessed. Photos will be examined for early microvascular changes and they may also provide information on potential neurodegenerative changes in the central nervous system.46
Bioelectrical impedance analysis
Body composition parameters such as fat mass, fat percentage, muscle mass, muscle mass percentage and fluid balance are measured by a bioelectrical impedance spectroscopy tool (SOZO). Almost as accurate as MRI and dual-energy X-ray absorptiometry, the method is easily applicable with low costs and provides a comprehensive set of values for body composition by distributing a weak electric current through the body tissues.47 48
Hand grip strength
HGS is a predictor of cardiovascular mortality,49–51 and ingestion of AAS has been associated with an increased HGS.52 HGS is measured using a hand dynamometer (Jamar Hand Dynamometer). Participants are seated with elbows by their side and flexed to right angles. In a neutral wrist position, the mean value of grip strength in three trials is calculated for each hand. The HGS procedure has been well documented as reliable in numerous studies.53 54 Participants’ HGS data will be displayed as left or right regardless of hand dominance.
Blood and urine
Circulating sex hormone levels and cardiovascular risk markers in serum/plasma (eg, HbA1c, lipids, haematocrit) are analysed along with inflammatory markers (high-sensitive) C reactive protein (hsCRP)). Hormone levels are measured by liquid chromatography tandem mass spectrometry, which is calibrated by in-house prepared calibrators, and the relative SD is <10%. Sex hormone binding globulin concentrations are measured by a Roche assay on Cobas e602 with a precision of 1.8 %−4.0% (14.9–21.9 nmol/L). Free testosterone levels are calculated assuming a plasma albumin concentration of 43 g/L.55
Haemoglobin is measured using a photometric analyser with a coefficient of variation (CV) of 2.8%. Plasma total cholesterol and high-density lipoprotein (HDL) cholesterol are analysed by enzymatic colorimetric reactions (Modular P, Roche), and low-density lipoprotein (LDL) cholesterol is calculated using the Friedewald equation.56 Hemoglobin A1c (HbA1c) is measured by high-performance liquid chromatography using Tosoh G8 (Medinor, Brøndby, Denmark); the analytical CV is 0.9%. CRP is analysed using latex-based immunoanalysis (CRP Ultra, Sentinel Diagnostics, Milan, Italy) by an Architect c8000 instrument (Abbott). The intra-assay and interassay CVs are 0.8% and 1.9% for normal levels of hsCRP, respectively.
Urine samples: Participants deliver a urine sample shortly after arrival. Urine samples are kept frozen at −80°C until analysis of AAS metabolites. Finally, biological materials (serum/plasma/urine) are stored for biobanking at −80°C.
Questionnaires
Our study employs a range of validated questionnaires to assess the mental health impacts of AAS use. SF-36 (Short Form Health Survey) assesses health-related QoL, providing insights into mental well-being, emotional health and social functioning.57 Inventory of Interpersonal Problems focuses on interpersonal difficulties, reflecting on how individuals perceive and handle relationship-related challenges.58 Body Q evaluates the subjective experience of one’s body and appearance, offering insights into body image concerns and psychological well-being.59 Buss-Perry Aggression Questionnaire measures dimensions of aggression, helping assess potential associations between AAS use and aggression.60 General Anxiety Disorder-7 quantifies anxiety symptoms, aiding in the identification of anxiety-related issues.61 Patient Health Questionnaire-9 is a depression screening tool, useful for measuring the severity of depressive symptoms.62 Beck’s Depression Inventory version II assesses the severity of depression, contributing valuable data on depressive symptoms in AAS users.63 Internet-based Cognition Assessment Tool evaluates cognitive function, providing insights into any cognitive effects or impairments associated with AAS use.64 By integrating these instruments, we aim to comprehensively evaluate specific mental health outcomes in AAS users.
Medical history
Chronic diseases, allergies, medication and supplement usage, alcohol and tobacco consumption, gynaecological history, sexual complaints and a comprehensive description of AAS history (including current or past use, cumulative dose and duration) will meticulously be documented. Additionally, information on the intake of other performance-enhancing drugs, dietary habits, physical activity and socioeconomic status is recorded.
Physical examination
We will conduct various anthropometric measurements, including the assessment of height, weight, waist and hip circumference. Additionally, BMI will be calculated to provide an indicator of body composition (expressed in kg/m²). Androgen-related features will be evaluated by estimating alopecia and assessing facial and body hair using the Ferriman-Gallwey score. Participants will self-report occurrences of acne. Moreover, testicular size will be measured using an orchidometer, contributing to a comprehensive assessment of physical characteristics and endocrine markers.
Sample size and statistics
It is not possible to perform a regular power calculation of the pilot study as the eligible study patients involve a highly diverse and broad group of recreational athletes with a lifetime illicit AAS use of different durations. Thus, our epidemiological study is including subjects having anything from a short period (ie, at least 3 months) of AAS use to subjects with a year lasting use of AAS. Due to this uncertainty regarding the composition of participants and the outcome of results, it is required to conduct a pilot project. In our pilot study (part A), we aim to include at least 120 participants divided between 90 males and 30 females with a history of AAS use. These individuals will be examined together with 60 physically active age-matched and sex-matched controls, who report no previous or ongoing illicit AAS use. Recruitment and inclusion of participants for the following cohort study (part B) will continue for another 2 years.
We will employ descriptive statistics to summarise demographic and clinical characteristics, presenting continuous variables as mean±SD or median (IQR) and categorical variables as number (n) and percentage (%). We will assess data distribution normality using Q-Q plots and the Shapiro-Wilk test, applying transformations as needed. For comparisons among the active AAS users, previous AAS users and controls, we will use one-way analysis of variance (ANOVA) for normally distributed continuous variables and χ2 tests for categorical variables. Post hoc analyses will be conducted following significant differences, with Levene’s test assessing homogeneity of variances. Kruskal-Wallis test will be applied for non-normally distributed variables. Correlation analyses will explore relationships, using Pearson’s correlation coefficient for normally distributed data and Spearman’s rank correlation coefficient for non-normally distributed data. To account for potential confounding variables, our future approach will involve multivariable regression models to estimate regression coefficients and associated 95% CIs for the primary outcomes.
Data management
Data are stored and analysed digitally with no unauthorised access. Original data are filed according to a participant number. Research Electronic Data Capture (REDCap) (www.project-redcap.org), hosted by Open Patient Data Explorative Network (OPEN), is used for registration of data.65 66 REDCap meets the safety requirements set by the Danish Data Protection Agency for the storage of person-sensible data. OPEN Analyse, a secure remote desktop solution hosted by OPEN, is used for storage and analyses of the pseudoanonymised data. OPEN Storage, a secure, logged drive also hosted by OPEN, will be used to store scans digitally.
Data are pseudoanonymised according to Danish law and regulations (The Regional Committees on Health Research Ethics for Southern Denmark, Project-ID: S-20210078, The Danish Data Protection Agency, journal no. 21/28259), and therefore, analyses will be performed through a remote VPN access to Statistics Denmark.
Ethics and dissemination
All participants are required to give written informed consent. The study results will be published in peer-reviewed international journals. Publication will be according to the International Committee of Medical Journal Editors recommendations, and the investigators oblige themselves to publish both positive and negative findings. All findings will also be presented at national and international conferences.
The study is performed in accordance with the Declaration of Helsinki and regulations of the General Data Protection Regulation. It is approved by the Regional Committee on Health Research Ethics for Southern Denmark (S-20210078) and the Danish Data Protection Agency (21/28259).
Discussion
Our study is specifically crafted to investigate the expanding and heterogeneous group of illicit AAS users, incorporating individuals with diverse fitness motivations, mental health challenges, different age groups, genders and social backgrounds. These individuals may have diverse objectives in their AAS use, with some seeking aesthetic enhancements, while others aim to sustain their mental well-being. For this reason, we intend to evaluate the cardiovascular and mental health status in a broad group of Danish recreational male and female athletes with an ongoing or previous use of AAS for at least 3 months. Given the broad nature of the target group, our approach involves conducting an initial pilot study (part A) with a comprehensive programme. Subsequently, we will use the gathered data to formulate an optimised and more focused programme for the larger cohort study (part B).
The available evidence regarding coronary atherosclerosis in AAS users originates from case reports, small observational studies14 and a few cross-sectional studies of long-term AAS users.16–18 Thus, the role of AAS in the development of atherosclerotic disease remains to be elucidated in the general population of recreational athletes with an illicit use of AAS.
Myocardial dysfunction as a consequence of AAS use seems to be somewhat better uncovered with a substantial amount of data collected.17 18 23 24 28 34 67 Nevertheless, as for atherosclerosis, most data are based on selected groups of AAS users (bodybuilders, weightlifters), and in this context, data on women using AAS are virtually absent. Thus, we expect our study to create new findings.
Ultrasonography of carotid arteries is a frequently used non-invasive approach to gain information regarding subclinical atherosclerosis,68 and indeed findings have been used to predict cardiovascular disease (CVD) outcome and atherosclerotic plaque burden in other vascular regions.19 69–72 Therefore, we found it of interest to include ultrasound of the carotid arteries in our pilot study. Finally, we will report new data on possible microvascular changes by comparing the morphology of retinal vasculature in AAS users and control subjects—something we do not believe has been examined previously.
In addition to cardiovascular data, we will incorporate supplementary information on mental health, acknowledging previous studies that have reported heightened levels of mental aggression and altered psychological well-being in AAS users.3 40 42 Whether this is a phenomenon that also concerns the general population of Danish AAS users is still unknown.
Because we specifically aim to target the general population of AAS users, our study has wide inclusion criteria, for example, age, AAS use (length of time, dose) and type and time spent on workout. While this approach is anticipated to facilitate participant recruitment, it introduces a potential challenge, as it becomes uncertain whether we primarily recruit young or middle-aged recreational athletes, individuals with current or previous AAS use, and those with a short or long-term history of AAS intake. To address this uncertainty and gain insight into the diverse composition of recreational athletes, we have chosen to conduct a pilot study (part A). In addition, this concept allows us to perform a comprehensive study programme including a large armamentarium of examinations, and subsequently to identify the examination modality (coronary CT with or without contrast) that appears to be the most applicable and useful to implement in our following cohort study (part B) of recreational athletes.
We will capitalise on the findings of the pilot study (part A) and create a down-scaled, but more specially designed and optimised screening programme. The examination modalities that effectively reveal the most obvious cardiovascular and mental health consequences of AAS use will subsequently form part of a larger cohort study (part B). Moreover, the cohort (part B) will undergo blood sampling, analyses of body composition, and answering questionnaires regarding mental aggression and QoL. The number of enrolled participants in the following CV cohort study (part B) is expected to be at least 300 male and female recreational athletes, and for practical and logistical reasons, this requires a less comprehensive examination programme.
Lastly, it is worth noting that a general limitation related to studies of AAS using recreational athletes is the inherent difficulty to obtain valid information regarding the use of different types of AAS, dosages and cycles, route of administration, training routines and the concurrent abuse of other substances and recreational drugs as these data in many cases are based on self-reported retrospective accounts far back in time.73 Consequently, it is not possible to completely assess and validate all data concerning the AAS use. To circumvent these limitations, and to encourage the participants to reveal a precise and detailed description of their AAS use and abuse of other substances, we keep all information strictly confidential, and no information is going to appear in the public medical records. Ethical permission is granted to access registry data from all participants.
bmjopen-2023-078558supp001.pdf (54.3KB, pdf)
Supplementary Material
Acknowledgments
We would like to thank our collaborators: Jakob Grauslund, Department of ophthalmology, Odense University Hospital, Odense, Denmark. Region of Southern Denmark for funding to DG and LLC ('Forskerkarrierepuljen').
Footnotes
@ADiederichsen
Contributors: We, the authors of this manuscript, hereby affirm that we have collectively met the criteria for authorship as outlined by the International Committee of Medical Journal Editors (ICMJE). JF, AD and MA: conception and design of the study. LFB, LLC, MA, DG, AD, JSL, CMK and JF contributed to writing the protocols for ethical approval or funding. This contributor statement attests to the fact that all authors have made substantial contributions to the conception, design, data acquisition, analysis, interpretation, drafting, critical revision and final approval of the manuscript. Furthermore, each author takes responsibility for the integrity of the work as a whole. AI (ChatGPT) was employed solely for the purpose of correcting grammar and errors within the manuscript.
Funding: The study is funded by the Novo Nordisk Foundation (to JF; grant number 0065138) and Anti Doping Denmark (To LLC and JF; no grant number).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
References
- 1. Sagoe D, Molde H, Andreassen CS, et al. The global epidemiology of anabolic-androgenic steroid use: a meta-analysis and meta-regression analysis. Ann Epidemiol 2014;24:383–98. 10.1016/j.annepidem.2014.01.009 [DOI] [PubMed] [Google Scholar]
- 2. Pope HG, Kanayama G, Athey A, et al. The lifetime prevalence of anabolic-androgenic steroid use and dependence in Americans: current best estimates. Am J Addict 2014;23:371–7. 10.1111/j.1521-0391.2013.12118.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gestsdottir S, Kristjansdottir H, Sigurdsson H, et al. Prevalence, mental health and substance use of anabolic steroid users: a population-based study on young individuals. Scand J Public Health 2021;49:555–62. 10.1177/1403494820973096 [DOI] [PubMed] [Google Scholar]
- 4. Pope HG, Wood RI, Rogol A, et al. Adverse health consequences of performance-enhancing drugs: an endocrine society scientific statement. Endocr Rev 2014;35:341–75. 10.1210/er.2013-1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rasmussen JJ, Selmer C, Østergren PB, et al. Former abusers of anabolic androgenic steroids exhibit decreased testosterone levels and hypogonadal symptoms years after cessation: a case-control study. PLoS One 2016;11:e0161208. 10.1371/journal.pone.0161208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. La Gerche A, Brosnan MJ. Cardiovascular effects of performance-enhancing drugs. Circulation 2017;135:89–99. 10.1161/CIRCULATIONAHA.116.022535 [DOI] [PubMed] [Google Scholar]
- 7. Horwitz H, Andersen JT, Dalhoff KP. Health consequences of androgenic anabolic steroid use. J Intern Med 2019;285:333–40. 10.1111/joim.12850 [DOI] [PubMed] [Google Scholar]
- 8. Merz AA, Cheng S. Sex differences in cardiovascular ageing. Heart 2016;102:825–31. 10.1136/heartjnl-2015-308769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Russell N, Grossmann M. Mechanisms in Endocrinology: estradiol as a male hormone. Eur J Endocrinol 2019;181:R23–43. 10.1530/EJE-18-1000 [DOI] [PubMed] [Google Scholar]
- 10. Glintborg D, Andersen M. Management of endocrine disease: morbidity in polycystic ovary syndrome. Eur J Endocrinol 2017;176:R53–65. 10.1530/EJE-16-0373 [DOI] [PubMed] [Google Scholar]
- 11. Ruth KS, Day FR, Tyrrell J, et al. Using human genetics to understand the disease impacts of testosterone in men and women. Nat Med 2020;26:252–8. 10.1038/s41591-020-0751-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Corona G, Rastrelli G, Di Pasquale G, et al. Testosterone and cardiovascular risk: meta-analysis of interventional studies. J Sex Med 2018;15:820–38. 10.1016/j.jsxm.2018.04.641 [DOI] [PubMed] [Google Scholar]
- 13. Albano GD, Amico F, Cocimano G, et al. Adverse effects of anabolic-androgenic steroids: a literature review. Healthcare (Basel) 2021;9:97. 10.3390/healthcare9010097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Santora LJ, Marin J, Vangrow J, et al. Coronary calcification in body builders using anabolic steroids. Prev Cardiol 2006;9:198–201. 10.1111/j.1559-4564.2006.05210.x [DOI] [PubMed] [Google Scholar]
- 15. Mitchell TL, Pippin JJ, Devers SM, et al. Age- and sex-based nomograms from coronary artery calcium scores as determined by electron beam computed tomography. Am J Cardiol 2001;87:453–6. 10.1016/s0002-9149(00)01403-x [DOI] [PubMed] [Google Scholar]
- 16. Souza F de, Dos Santos MR, Porello RA, et al. Diminished cholesterol efflux mediated by HDL and coronary artery disease in young male anabolic androgenic steroid users. Atherosclerosis 2019;283:100–5. 10.1016/j.atherosclerosis.2019.02.006 [DOI] [PubMed] [Google Scholar]
- 17. Baggish AL, Weiner RB, Kanayama G, et al. Cardiovascular toxicity of illicit anabolic-androgenic steroid use. Circulation 2017;135:1991–2002. 10.1161/CIRCULATIONAHA.116.026945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fyksen TS, Vanberg P, Gjesdal K, et al. The cardiovascular phenotype of long-term anabolic-androgenic steroid abusers compared to strength-trained athletes. Scand J Med Sci Sports 2022;32:1170–81. 10.1111/sms.14172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Belcaro G, Nicolaides AN, Ramaswami G, et al. Carotid and femoral ultrasound morphology screening and cardiovascular events in low risk subjects: a 10-year follow-up study (the CAFES-CAVE study(1)). Atherosclerosis 2001;156:379–87. 10.1016/s0021-9150(00)00665-1 [DOI] [PubMed] [Google Scholar]
- 20. Sosnowski C, Pasierski T, Janeczko-Sosnowska E, et al. Femoral rather than carotid artery ultrasound imaging predicts extent and severity of coronary artery disease. Kardiol Pol 2007;65:760–6. [PubMed] [Google Scholar]
- 21. Damasceno EF, Neto AM, Damasceno NAP, et al. Branch retinal vein occlusion and anabolic steroids abuse in young bodybuilders. Acta Ophthalmol 2009;87:580–1. 10.1111/j.1755-3768.2008.01238.x [DOI] [PubMed] [Google Scholar]
- 22. Lippi G, Banfi G. Doping and thrombosis in sports. Semin Thromb Hemost 2011;37:918–28. 10.1055/s-0031-1297371 [DOI] [PubMed] [Google Scholar]
- 23. Rasmussen JJ, Schou M, Madsen PL, et al. Cardiac systolic dysfunction in past illicit users of anabolic androgenic steroids. Am Heart J 2018;203:49–56. 10.1016/j.ahj.2018.06.010 [DOI] [PubMed] [Google Scholar]
- 24. Luijkx T, Velthuis BK, Backx FJG, et al. Anabolic androgenic steroid use is associated with ventricular dysfunction on cardiac MRI in strength trained athletes. Int J Cardiol 2013;167:664–8. 10.1016/j.ijcard.2012.03.072 [DOI] [PubMed] [Google Scholar]
- 25. Grandperrin A, Schuster I, Rupp T, et al. Left ventricular dyssynchrony and post-systolic shortening in young bodybuilders using anabolic-androgenic steroids. Am J Physiol Heart Circ Physiol 2021;321:H509–17. 10.1152/ajpheart.00136.2021 [DOI] [PubMed] [Google Scholar]
- 26. Grandperrin A, Schuster I, Moronval P, et al. Anabolic steroids use is associated with impairments in atrial and ventricular cardiac structure and performance in athletes. Med Sci Sports Exerc 2022;54:780–8. 10.1249/MSS.0000000000002852 [DOI] [PubMed] [Google Scholar]
- 27. Kouidi EJ, Kaltsatou A, Anifanti MA, et al. Early left ventricular diastolic dysfunction, reduced Baroreflex sensitivity, and cardiac autonomic imbalance in anabolic-androgenic steroid users. Int J Environ Res Public Health 2021;18:6974. 10.3390/ijerph18136974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. D’Andrea A, Caso P, Salerno G, et al. Left ventricular early myocardial dysfunction after chronic misuse of anabolic androgenic steroids: a doppler myocardial and strain imaging analysis. Br J Sports Med 2007;41:149–55. 10.1136/bjsm.2006.030171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Darke S, Torok M, Duflou J. Sudden or unnatural deaths involving anabolic-androgenic steroids. J Forensic Sci 2014;59:1025–8. 10.1111/1556-4029.12424 [DOI] [PubMed] [Google Scholar]
- 30. Far HRM, Ågren G, Thiblin I. Cardiac hypertrophy in deceased users of anabolic androgenic steroids: an investigation of autopsy findings. Cardiovasc Pathol 2012;21:312–6. 10.1016/j.carpath.2011.10.002 [DOI] [PubMed] [Google Scholar]
- 31. Thiblin I, Lindquist O, Rajs J. Cause and manner of death among users of anabolic androgenic steroids. J Forensic Sci 2000;45:16–23. [PubMed] [Google Scholar]
- 32. Achar S, Rostamian A, Narayan SM. Cardiac and metabolic effects of anabolic-androgenic steroid abuse on lipids, blood pressure, left ventricular dimensions, and rhythm. Am J Cardiol 2010;106:893–901. 10.1016/j.amjcard.2010.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Baggish AL, Weiner RB, Kanayama G, et al. Long-term anabolic-androgenic steroid use is associated with left ventricular dysfunction. Circ Heart Fail 2010;3:472–6. 10.1161/CIRCHEARTFAILURE.109.931063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Smit DL, Buijs MM, de Hon O, et al. Positive and negative side effects of androgen abuse. The HAARLEM study: a one-year prospective cohort study in 100 men. Scand J Med Sci Sports 2021;31:427–38. 10.1111/sms.13843 [DOI] [PubMed] [Google Scholar]
- 35. Vanberg P, Atar D. Androgenic anabolic steroid abuse and the cardiovascular system. Handb Exp Pharmacol 2010;2010:411–57. 10.1007/978-3-540-79088-4_18 [DOI] [PubMed] [Google Scholar]
- 36. D’Ascenzo S, Millimaggi D, Di Massimo C, et al. Detrimental effects of anabolic steroids on human endothelial cells. Toxicol Lett 2007;169:129–36. 10.1016/j.toxlet.2006.12.008 [DOI] [PubMed] [Google Scholar]
- 37. Westlye LT, Kaufmann T, Alnæs D, et al. Brain connectivity aberrations in anabolic-androgenic steroid users. Neuroimage Clin 2017;13:62–9. 10.1016/j.nicl.2016.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kanayama G, Barry S, Hudson JI, et al. Body image and attitudes toward male roles in anabolic-androgenic steroid users. Am J Psychiatry 2006;163:697–703. 10.1176/ajp.2006.163.4.697 [DOI] [PubMed] [Google Scholar]
- 39. Windfeld-Mathiasen J, Christoffersen T, Strand NAW, et al. Psychiatric morbidity among men using anabolic steroids. Depress Anxiety 2022;39:805–12. 10.1002/da.23287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nelson BS, Hildebrandt T, Wallisch P. Anabolic-androgenic steroid use is associated with psychopathy, risk-taking, anger, and physical problems. Sci Rep 2022;12:9133. 10.1038/s41598-022-13048-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pope HG, Kanayama G, Ionescu-Pioggia M, et al. Anabolic steroid users' attitudes towards physicians. Addiction 2004;99:1189–94. 10.1111/j.1360-0443.2004.00781.x [DOI] [PubMed] [Google Scholar]
- 42. Pope HG, Kanayama G, Hudson JI, et al. Review article: anabolic-androgenic steroids, violence, and crime. Am J Addict 2021;30:423–32. 10.1111/ajad.13157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Otaki Y, Gransar H, Cheng VY, et al. Gender differences in the prevalence, severity, and composition of coronary artery disease in the young: a study of 1635 individuals undergoing coronary CT angiography from the prospective, multinational confirm registry. Eur Heart J Cardiovasc Imaging 2015;16:490–9. 10.1093/ehjci/jeu281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dey D, Gaur S, Ovrehus KA, et al. Integrated prediction of lesion-specific ischaemia from quantitative coronary CT angiography using machine learning: a multicentre study. Eur Radiol 2018;28:2655–64. 10.1007/s00330-017-5223-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Parkkila K, Kesäniemi YA, Ukkola O. Comparing ultrasonographically assessed carotid and abdominal aorta plaques in cardiovascular disease risk estimation. BMC Cardiovasc Disord 2023;23:245. 10.1186/s12872-023-03264-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yoon SP, Grewal DS, Thompson AC, et al. Retinal microvascular and neurodegenerative changes in Alzheimer’s disease and mild cognitive impairment compared with control participants. Ophthalmology Retina 2019;3:489–99. 10.1016/j.oret.2019.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dehghan M, Merchant AT. Is bioelectrical impedance accurate for use in large epidemiological studies? Nutr J 2008;7:26. 10.1186/1475-2891-7-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Moonen HPFX, Van Zanten ARH. Bioelectric impedance analysis for body composition measurement and other potential clinical applications in critical illness. Curr Opin Crit Care 2021;27:344–53. 10.1097/MCC.0000000000000840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Leong DP, Teo KK, Rangarajan S, et al. Prognostic value of grip strength: findings from the prospective urban rural epidemiology (PURE) study. Lancet 2015;386:266–73. 10.1016/S0140-6736(14)62000-6 [DOI] [PubMed] [Google Scholar]
- 50. Bohannon RW. Grip strength: an indispensable biomarker for older adults. Clin Interv Aging 2019;14:1681–91. 10.2147/CIA.S194543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Celis-Morales CA, Welsh P, Lyall DM, et al. Associations of grip strength with cardiovascular, respiratory, and cancer outcomes and all cause mortality: prospective cohort study of half a million UK biobank participants. BMJ 2018;361:k1651. 10.1136/bmj.k1651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Supasyndh O, Satirapoj B, Aramwit P, et al. Effect of oral anabolic steroid on muscle strength and muscle growth in hemodialysis patients. Clin J Am Soc Nephrol 2013;8:271–9. 10.2215/CJN.00380112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hamilton GF, McDonald C, Chenier TC. Measurement of grip strength: validity and reliability of the sphygmomanometer and Jamar grip dynamometer. J Orthop Sports Phys Ther 1992;16:215–9. 10.2519/jospt.1992.16.5.215 [DOI] [PubMed] [Google Scholar]
- 54. Roberts HC, Denison HJ, Martin HJ, et al. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing 2011;40:423–9. 10.1093/ageing/afr051 [DOI] [PubMed] [Google Scholar]
- 55. Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 1999;84:3666–72. 10.1210/jcem.84.10.6079 [DOI] [PubMed] [Google Scholar]
- 56. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499–502. [PubMed] [Google Scholar]
- 57. Ware JE, Gandek B. Overview of the SF-36 health survey and the international quality of life assessment (IQOLA). J Clin Epidemiol 1998;51:903–12. 10.1016/s0895-4356(98)00081-x [DOI] [PubMed] [Google Scholar]
- 58. Horowitz LM, Rosenberg SE, Baer BA, et al. Inventory of interpersonal problems: psychometric properties and clinical applications. J Consult Clin Psychol 1988;56:885–92. 10.1037//0022-006x.56.6.885 [DOI] [PubMed] [Google Scholar]
- 59. Klassen AF, Cano SJ, Alderman A, et al. The BODY-Q: a patient-reported outcome instrument for weight loss and body contouring treatments. Plast Reconstr Surg Glob Open 2016;4:e679. 10.1097/GOX.0000000000000665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Buss AH, Perry M. The aggression questionnaire. J Pers Soc Psychol 1992;63:452–9. 10.1037//0022-3514.63.3.452 [DOI] [PubMed] [Google Scholar]
- 61. Spitzer RL, Kroenke K, Williams JBW, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 2006;166:1092–7. 10.1001/archinte.166.10.1092 [DOI] [PubMed] [Google Scholar]
- 62. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606–13. 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Steer RA, Ball R, Ranieri WF, et al. Dimensions of the beck depression inventory-II in clinically depressed outpatients. J Clin Psychol 1999;55:117–28. [DOI] [PubMed] [Google Scholar]
- 64. Hafiz P, Miskowiak KW, Kessing LV, et al. The internet-based cognitive assessment tool: system design and feasibility study. JMIR Form Res 2019;3:e13898. 10.2196/13898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Harris PA, Taylor R, Minor BL, et al. The REDcap consortium: building an international community of software platform partners. J Biomed Inform 2019;95:103208. 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDcap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Smit DL, de Hon O, Venhuis BJ, et al. Baseline characteristics of the HAARLEM study: 100 male amateur athletes using anabolic androgenic steroids. Scand J Med Sci Sports 2020;30:531–9. 10.1111/sms.13592 [DOI] [PubMed] [Google Scholar]
- 68. Touboul P-J, Grobbee DE, den Ruijter H. Assessment of subclinical atherosclerosis by carotid intima media thickness: technical issues. Eur J Prev Cardiol 2012;19:18–24. 10.1177/2047487312448990 [DOI] [PubMed] [Google Scholar]
- 69. Bytyçi I, Shenouda R, Wester P, et al. Carotid atherosclerosis in predicting coronary artery disease: a systematic review and meta-analysis. Arterioscler Thromb Vasc Biol 2021;41:e224–37. 10.1161/ATVBAHA.120.315747 [DOI] [PubMed] [Google Scholar]
- 70. Sillesen H, Sartori S, Sandholt B, et al. Carotid plaque thickness and carotid plaque burden predict future cardiovascular events in asymptomatic adult Americans. Eur Heart J Cardiovasc Imaging 2018;19:1042–50. 10.1093/ehjci/jex239 [DOI] [PubMed] [Google Scholar]
- 71. Polak JF, Szklo M, Kronmal RA, et al. The value of carotid artery plaque and intima-media thickness for incident cardiovascular disease: the multi-ethnic study of atherosclerosis. J Am Heart Assoc 2013;2:e000087. 10.1161/JAHA.113.000087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Razzouk L, Rockman CB, Patel MR, et al. Co-existence of vascular disease in different arterial beds: peripheral artery disease and carotid artery stenosis--data from life line screening((R)). Atherosclerosis 2015;241:687–91. 10.1016/j.atherosclerosis.2015.06.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Parkinson AB, Evans NA. Anabolic androgenic steroids: a survey of 500 users. Med Sci Sports Exerc 2006;38:644–51. 10.1249/01.mss.0000210194.56834.5d [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-078558supp001.pdf (54.3KB, pdf)