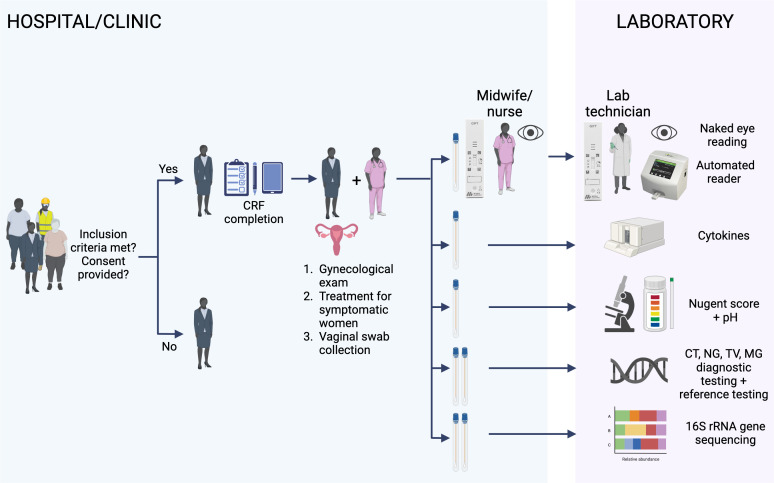
Figure 1.
Schematic of the GIFT diagnostic study. The recruited women who meet the inclusion criteria and consent to participate in the GIFT study will be administered a questionnaire. Data will be recorded on CRFs in a paper or electronic (tablet) format by the clinical nurse followed by a gynaecological examination. Vaginal swabs are collected: the first collected swab will be used for two GIFT assays; the first GIFT will be performed at the bedside by the midwife/nurse and the second GIFT by the lab technician. Each of them will read visually their own performed GIFT (naked eye reading). The two respective GIFT assays will then be read using a lateral flow automated reader. The other collected swabs will be used for different laboratory assays for the evaluation of the GIFT performance. HIV testing on fingerpick blood will be also included at the end of the medical examination. The figure was created with BioRender. CRFs, case report forms; CT, Chlamydia trachomatis; GIFT, Genital InFlammation Test; MG, Mycoplasma genitalium; NG, Neisseria gonorrhoeae; TV, Trichomonas vaginalis.