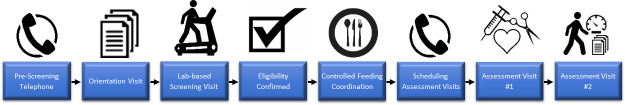
Figure 3.
Participant screening, enrolment and baseline assessment. A pre-screening telephone interview determines the potential eligibility of the participant. The orientation visit includes the completion of administrative forms, laboratory-based screening informed consent and release forms for obtaining medical clearance. Once medical clearance is received by the study team, the participant completes the laboratory-based screening visit, which includes collecting V̇O2peak and body mass index. If deemed eligible based on the screening visit, the individual will be invited to sign the consent for full study participation and be scheduled for controlled feeding initiation. Baseline assessment visit #1 is scheduled for at least 1 week after initiation of controlled feeding. Within 7 days of visit #1, (1) the participant is asked to collect the faecal sample at home 2–3 days after visit #1 and promptly overnight ships it to the laboratory, and then (2) complete the remaining assessment materials (eg, fatigue survey) 2–3 days after collecting the faecal sample and baseline visit #2 occurs to return these forms.