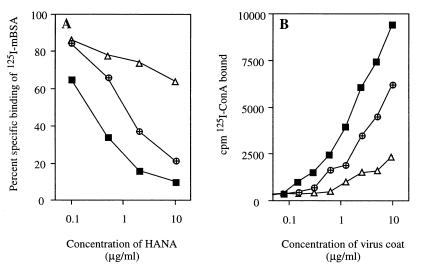
FIG. 2.
Relationship between the relative binding avidity of the virus HANA glycoproteins for the MR on peritoneal Mφ and the content of high-mannose and hybrid-type oligosaccharide on influenza virus as determined by 125I-ConA binding. The virus strains used were BJx109 (■), HKx31 (⊕), and PR8 (▵). (A) Ability of HANA preparations of the three viruses to inhibit the binding of 125I-mBSA to peritoneal Mφ. (B) Binding of 125I-ConA to microtiter wells coated with increasing concentrations of purified influenza viruses. Binding was completely inhibited in the presence of 100 mM α-methylmannoside (data not shown). Coating levels of the three viruses were confirmed to be similar by using MAbs to the viral NP and to the host-derived carbohydrate antigen common to all egg-grown strains of influenza virus, as described in Materials and Methods (data not shown).