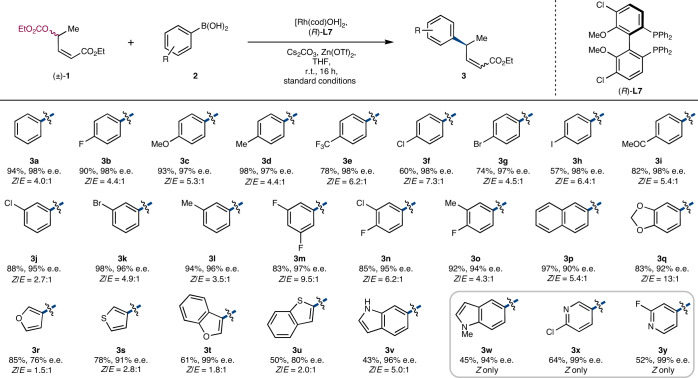
Table 2.
Scope of boronic acids
Reaction conditions were as follows: [Rh(cod)OH]2 (2.5 mol%), L7 (6.0 mol%), (±)-1 (0.4 mmol, 1.0 equiv.), 2 (2.0 equiv.), Cs2CO3 (1.0 equiv.), Zn(OTf)2 (20 mol%), THF (0.1 M), r.t., 14 h. All experiments were performed on the 0.4 mmol scale. All compounds were isolated as single regioisomers (r.r. >99:1). Z/E ratios were determined by 1H NMR spectroscopy on crude reaction mixtures. Yields were determined by subsequent hydrogenation of the product mixture. The e.e. values were determined by hydrogenation of the product mixture and SFC analysis using a chiral non-racemic stationary phase. Absolute configurations were assigned by analogy to product 3c, which was converted to (S)-curcumene as determined by comparing optical rotation values to those previously reported.