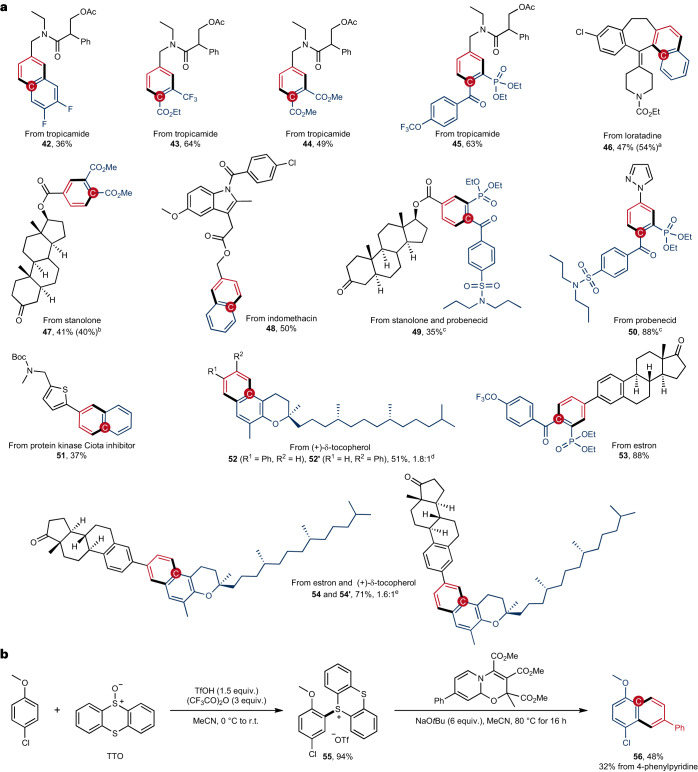
Fig. 2. Application of the skeletal editing strategy.
a, Skeletal editing of pyridine cores in drugs and drug derivatives. Compounds 42, 46, 48, 51, 52, 54 are obtained under condition A, whereas others are synthesized under condition B. All yields are isolated yields based on pyridine in a one-pot process unless stated otherwise. aThe gram-scale yield (in parenthesis) by using condition A. bThe gram-scale yield (in parenthesis) by using condition B under air. cApplying condition B with probenecid-derived alkyne (1.2 equiv.); the yield is based on the pyridine. dWith condition A using (+)-δ-tocopherol derived aryne precursor (1 equiv.), 4-phenylpyridine (1.5 equiv.), DMAD (1.5 equiv.), MP (1.5 equiv.) and caesium fluoride (1.5 equiv.); the yield is based on the aryne precursor. eCombined yield of the two constitutional isomers. b, Application of aryne formation from aryl thianthrenium salt in pyridine editing.