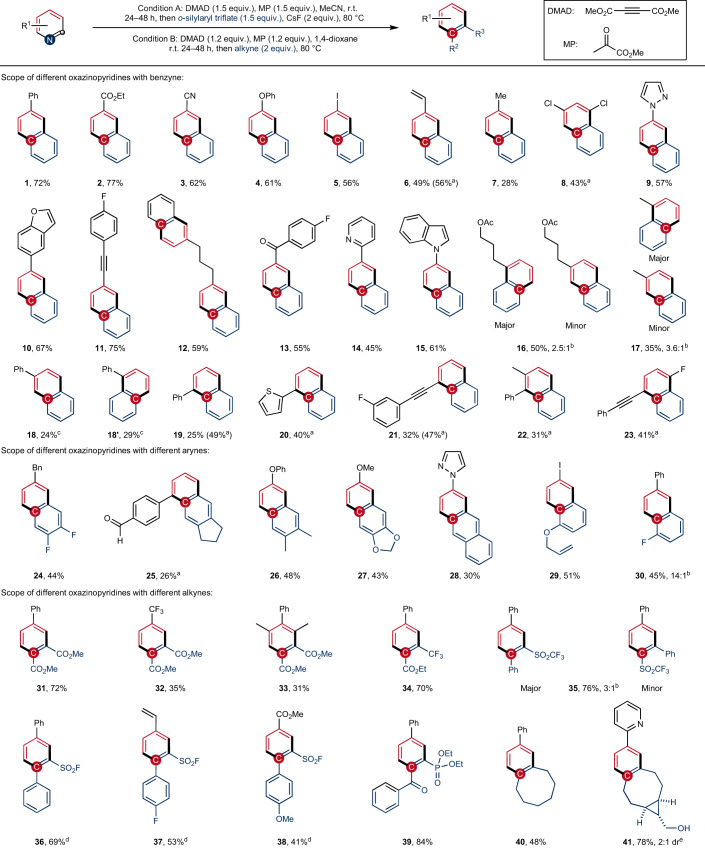
Table 1.
Scope of pyridine skeletal editing
Compounds 1–30 are obtained under condition A, in which ortho-trimethylsilylaryl triflates are used as aryne precursors. Under condition B, multisubstituted benzenes 31–41 are generated from reactions with activated alkynes. All yields are isolated yields based on pyridines in a one-pot process, unless stated otherwise. aYield based on the isolated oxazino pyridine intermediates. bCombined yield of two constitutional isomers. cTwo isolable constitutional isomers, with a ratio of 1.2:1. dApplying condition B, using toluene as a solvent instead of 1,4-dioxane. eThe cyclooctyne was used as a 2:1 diastereoisomeric mixture (see page 77 of the Supplementary Information). DMAD, dimethyl acetylenedicarboxylate; MP, methyl pyruvate.