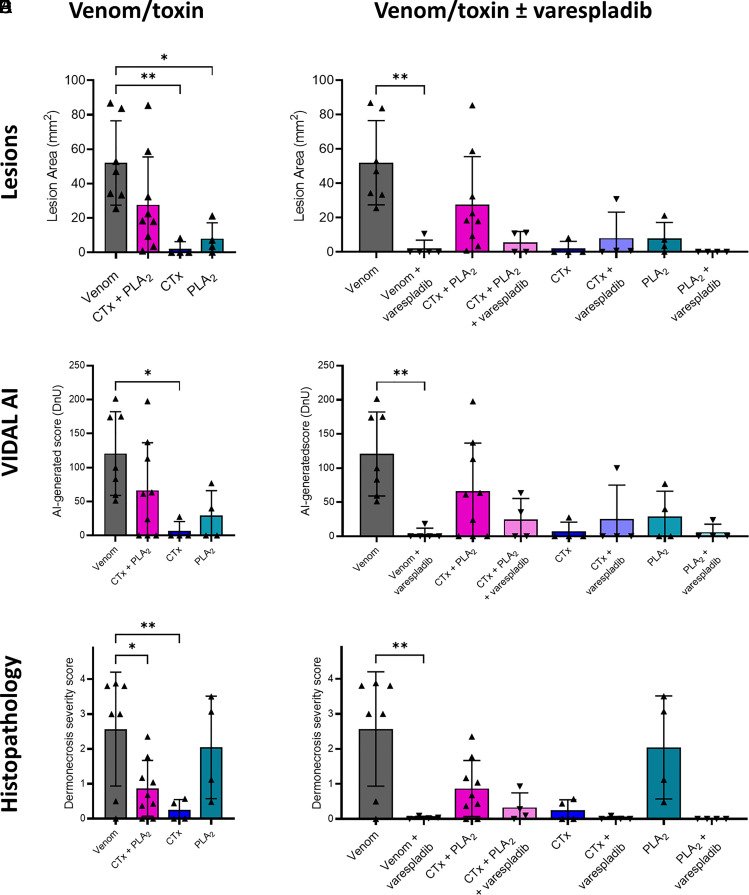
Fig. 3.
Spitting cobra venom causes dermonecrosis in vivo via CTx and PLA2 toxin potentiation, and inhibition of PLA2 toxins with varespladib reduces dermonecrosis severity. Groups of mice (n ≥ 4) were intradermally injected with either East African (Tanzania) N. nigricollis venom or purified venom constituents (CTx, PLA2, or a combination of CTx + PLA2) at doses reflecting their relative abundance in crude venom, with or without the PLA2-inhibiting small molecule drug varespladib (19 μg). At 72 h postinjection, lesions were excised and examined macroscopically and histopathologically. (A) A combination of venom CTx and PLA2 was required to recapitulate the dermonecrotic activity of crude N. nigricollis venom, as CTx and PLA2 toxins alone did not cause extensive dermonecrosis, as quantified via (A) caliper-measurements of lesion height and width, and (B) the lesion severity measuring AI tool, VIDAL. (C) Histopathological analysis of excised lesions showed similar results, except for a more severe dermonecrotic effect of the PLA2 alone. Preincubation with the PLA2 inhibitor varespladib reduced dermonecrotic lesion severity caused by East African N. nigricollis venom with similar trends for CTx + PLA2 and PLA2, as quantified with (D) calipers, (E) VIDAL, and (F) histopathological analysis. For damage scores of individual skin layers, see SI Appendix, Fig. S10. For panels A and D, the data shown represent mean lesion areas and corresponding SDs. Panels B and E show the mean lesion severity, as determined by VIDAL. Panels C and F show mean dermonecrosis severity scores and corresponding SDs calculated from those of the individual skin layers (SI Appendix, Fig. S10). Statistically significant differences were determined by one-way ANOVAs followed by Tukey's multiple comparisons post hoc tests and are denoted by asterisks: *P < 0.05, **P < 0.01. Error bars represent SDs.