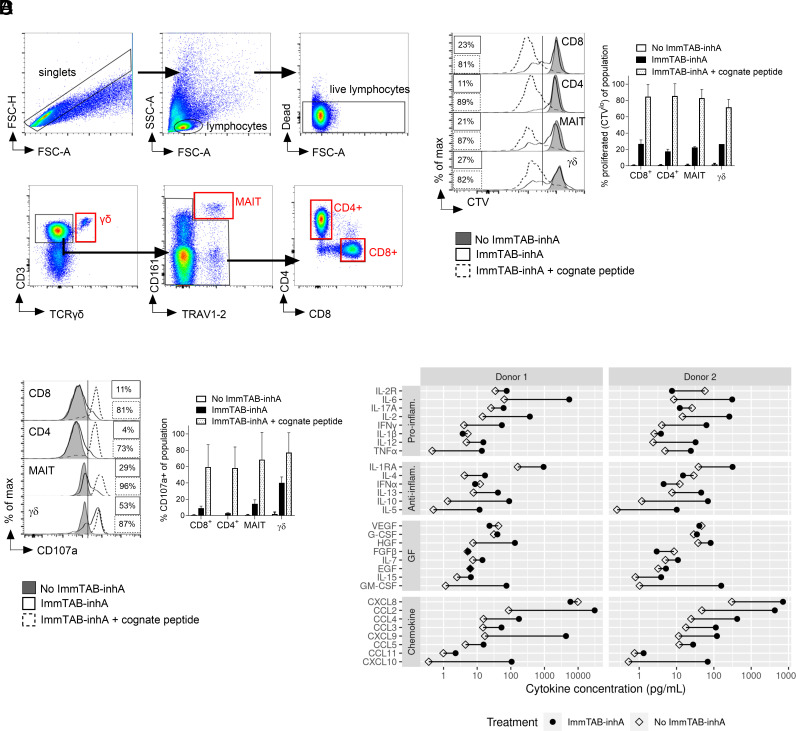
Fig. 5.
ImmTAB-inhA redirects multiple T cell subsets to elicit polyfunctional responses. (A) Gating strategy for identification of T cell subsets. T cell subsets were identified by gating first on singlets (FSC-A vs. FSC-H) and then lymphocytes (FSC-A vs. SSC-A). Live lymphocytes were then gated as cells excluding the fixable viability dye. From this negative gate, γδ T cells were identified as CD3+TCRγδ+. From the CD3+ TCRγδ− gate, MAIT cells were identified as CD161+TRAV1-2+. The remaining cells were divided into CD4+CD8− (CD4+ T cells) or CD4−CD8+ (CD8+ T cells). (B and C) Flow cytometry analysis of (B) CTV dilution and (C) CD107a+ surface levels induced by 1 nM ImmTAB-inhA in indicated T cell subsets identified by specific gating within PBMC cocultured with inhA-transduced HEK293T cells. The same analysis was performed within parallel control cocultures not treated with ImmTAB-inhA (no ImmTAB-inhA) or supplemented with ImmTAB-inhA and the cognate peptide (ImmTAB-inhA + cognate peptide). Left panels show representative histogram plots. Right panels depict cumulative results from four donors. Data in the Right panels are plotted as mean ± SD. (D) ImmTAB-inhA induces production of proinflammatory cytokines. PBMC effectors (E) were incubated with Ag+ HEK 293T target cells (T) at a 10:1 ratio in the absence or presence of 1 nM ImmTAB-inhA (I) for 5 d. Production of cytokines (proinflammatory and anti-inflammatory), GF, and chemokines was assessed in culture supernatants by the Luminex assay. The concentration of each cytokine is plotted for cultures without (E+T; open diamonds) and with ImmTAB-inhA (E+T+I; black circles) for each donor tested (n = 2).