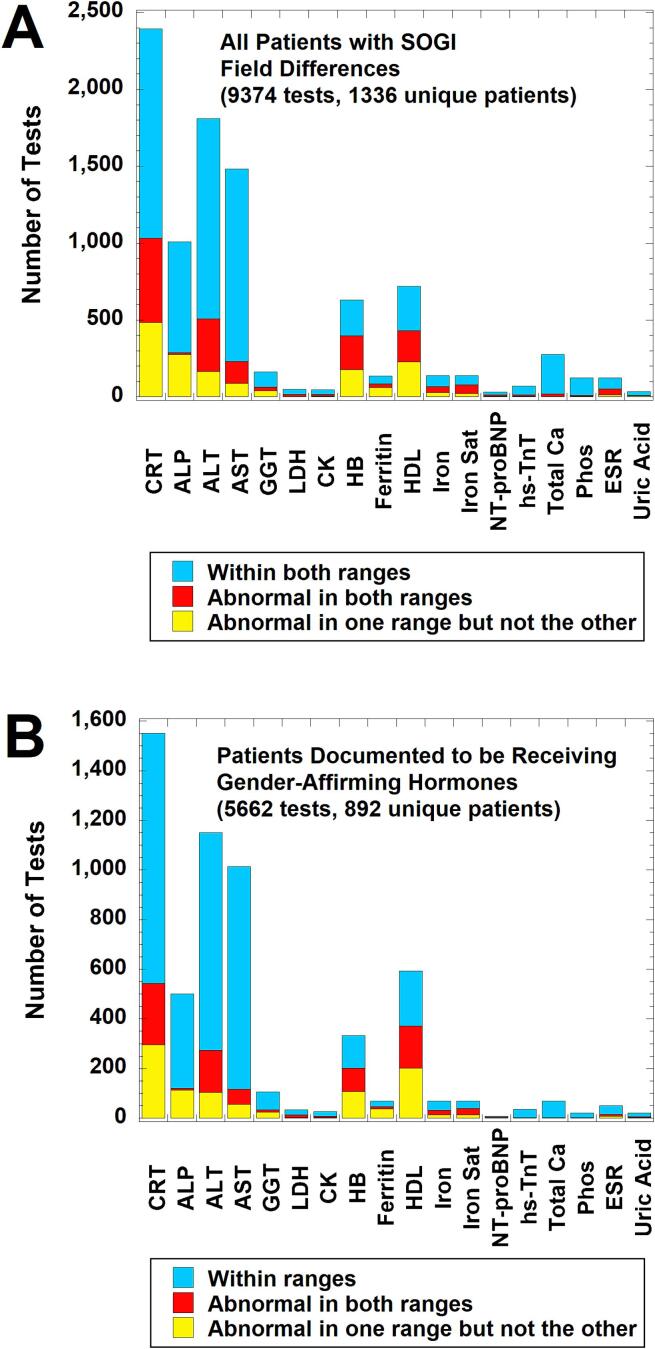
Fig. 2.
Laboratory tests with sex-specific reference intervals (RIs) ordered in calendar year 2021 on patients with sexual orientation/gender identity (SOGI) field differences for legal sex, sex assigned at birth, and gender identity. The bar graphs indicate how often laboratory tests were within sex-specific RIs for both sexes (light blue), abnormal in both intervals (red), or abnormal in only one sex-specific interval (yellow) using the patient age at time of laboratory testing. The sex-specific RIs were the ones used by the laboratory for all patients (see Supplemental Table 2), not any RIs empirically derived for the population on gender-affirming therapy. (A) Analysis for all patients (9374 tests on 1336 unique patients). (B) Analysis for the subset of patients documented to be receiving gender-affirming hormones (either estradiol or testosterone) at the time of laboratory testing (5662 tests on 892 unique patients). Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; CK, creatine kinase; CRT, creatinine; ESR, erythrocyte sedimentation rate; GGT, gamma-glutamyltransferase; HB, hemoglobin; HDL, high-density lipoprotein; hs-TnT, high-sensitivity troponin T; Iron Sat, iron saturation %; LDH, lactate dehydrogenase; NT-proBNP, NT-Pro B-type natriuretic peptide; Phos, inorganic phosphorus; SOGI, sexual orientation/gender identity. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)