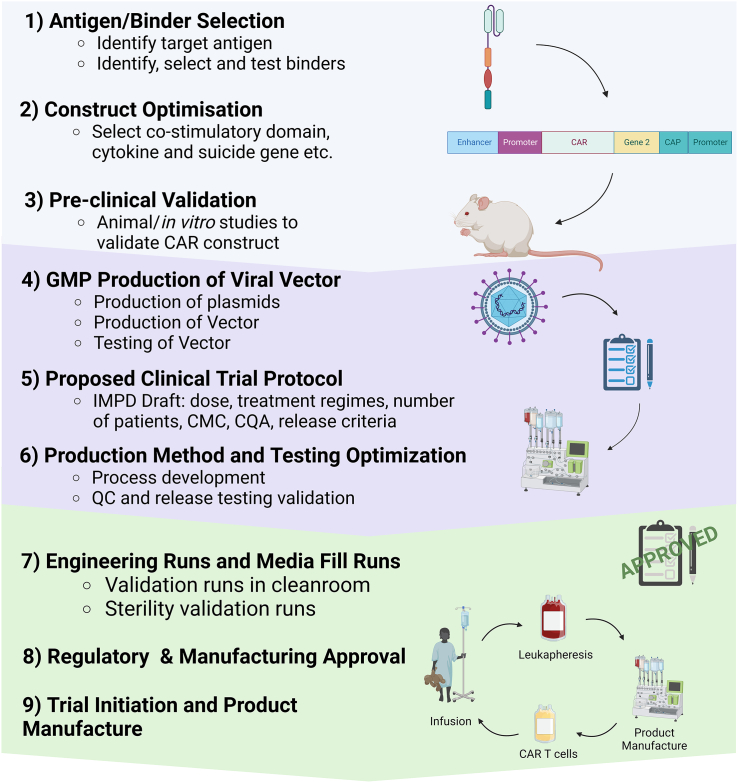
Figure 2.
Bench-to-bedside workflow used in the UCL program for translation and delivery of new phase 1 CAR-T cell studies
New target antigens and specific binders are identified in the research lab and CAR constructs optimized for the desired application (e.g., co-stimulatory domains, cytokine secretion, suicide or marker genes, etc.). The chosen CARs are validated in vitro and with the use of animal models and, if applicable, genome-editing strategies are also validated. A viral vector batch is then manufactured under GMP compliance. At this stage, it is expected that a proposed trial protocol is available, indicating the treatment regimens and required doses, as this will help defining the critical quality attributes of the drug product. Based on this information, the proposed manufacturing protocol is adjusted, a testing plan is devised, and testing methods optimized, before scale-up engineering runs are completed to show that products can be manufactured with the desired characteristics. All data on literature review and trial rationale, ethical considerations, risk-benefit analysis, pre-clinical validation data, detailed manufacturing methods and compliance, drug product characteristics, and proposed protocol are submitted for regulatory review by the MHRA in the UK. Once a CTA is granted, internal protocols are followed to complete the required validations and obtain the relevant approvals. The team is then trained on the pre-approved manufacturing and testing methods and products are manufacture in accordance with issued SOPs. Each batch manufactured undergoes detailed review by the quality team and the Qualified Person (QP) prior to release and results are constantly monitored to continuously improve processes. CAR, chimeric antigen receptors; CMC, chemistry, manufacturing, and control; CQA, critical quality attributes; QC, quality control; cGMP, current good manufacturing practice; MHRA, Medicines and Healthcare Products Regulatory Agency; CTA, clinical trial authorization.