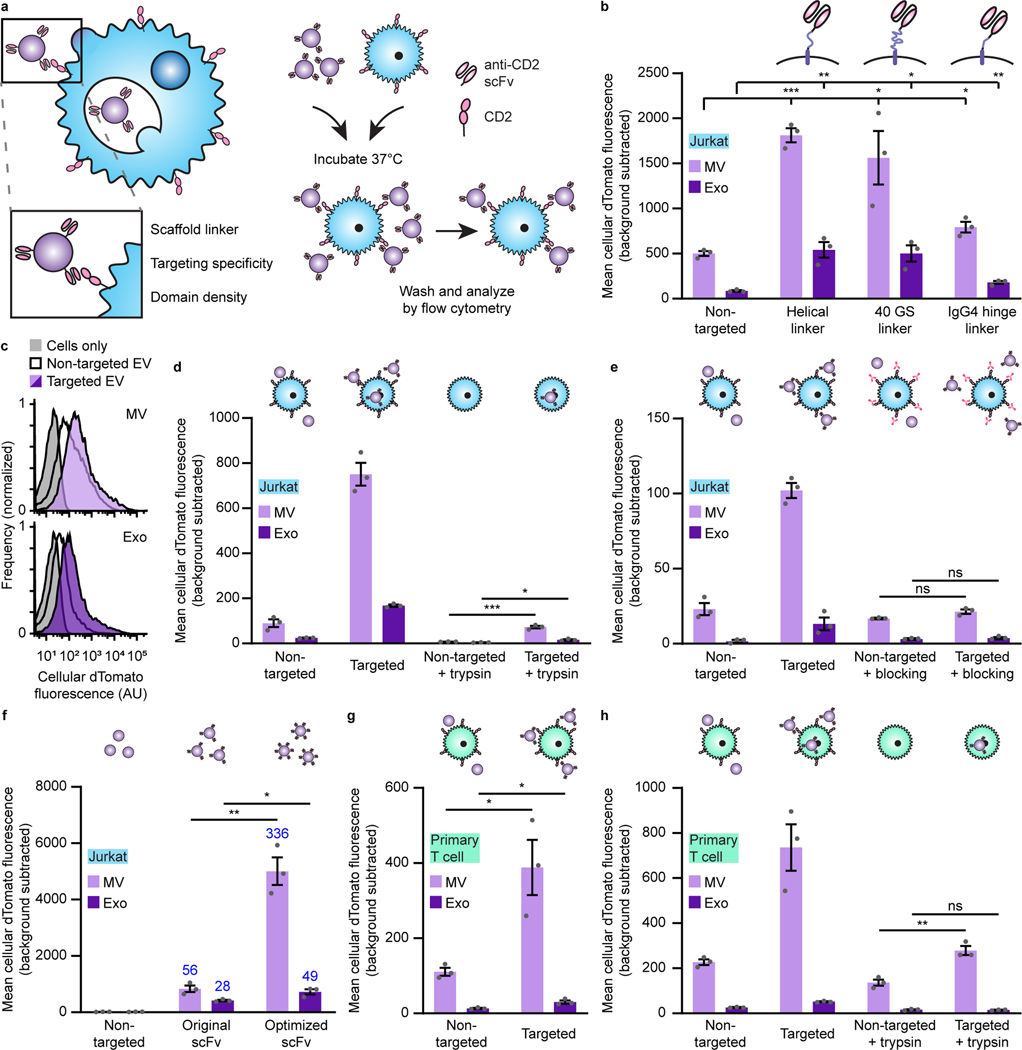
Fig. 2: Display of scFvs on EVs mediates receptor-specific, targeted binding and uptake to T cells.
a, Strategy for targeting EVs to T cells (left) and illustration of EV binding experiments (right). b, Targeted EVs binding to Jurkats (2 h incubation). To evaluate potential differences in dTomato loading, average EV fluorescence was analyzed separately (Supplementary Fig. 5). c, Representative histograms depicting distributions of helical linker EV-mediated fluorescence in recipient cells analyzed in b. d, Distinguishing binding and internalization for EVs targeted to Jurkats. Trypsinization was used to remove bound, non-internalized EVs following a 6 h incubation. e, Specificity of EV targeting to CD2. Pre-incubation with anti-CD2 antibodies ablated EV targeting to Jurkats. f, Enhancement of targeting by codon-optimized expression of scFv constructs. Fold increases over the non-targeted control are reported in blue. g, Binding of targeted EVs to primary human CD4+ T cells (2 h incubation). h, Distinguishing binding and internalization for EVs targeted to primary human CD4+ T cells. All experiments were performed in biological triplicate, and error bars indicate standard error of the mean. Statistical tests comprise two-tailed Student’s t-tests using the Benjamini-Hochberg method to reduce the false discovery rate. (*p < 0.05, **p < 0.01, ***p < 0.001). Exact p-values are reported in Supplementary Table 1. EV dTomato loading evaluations are in Supplementary Fig. 5.