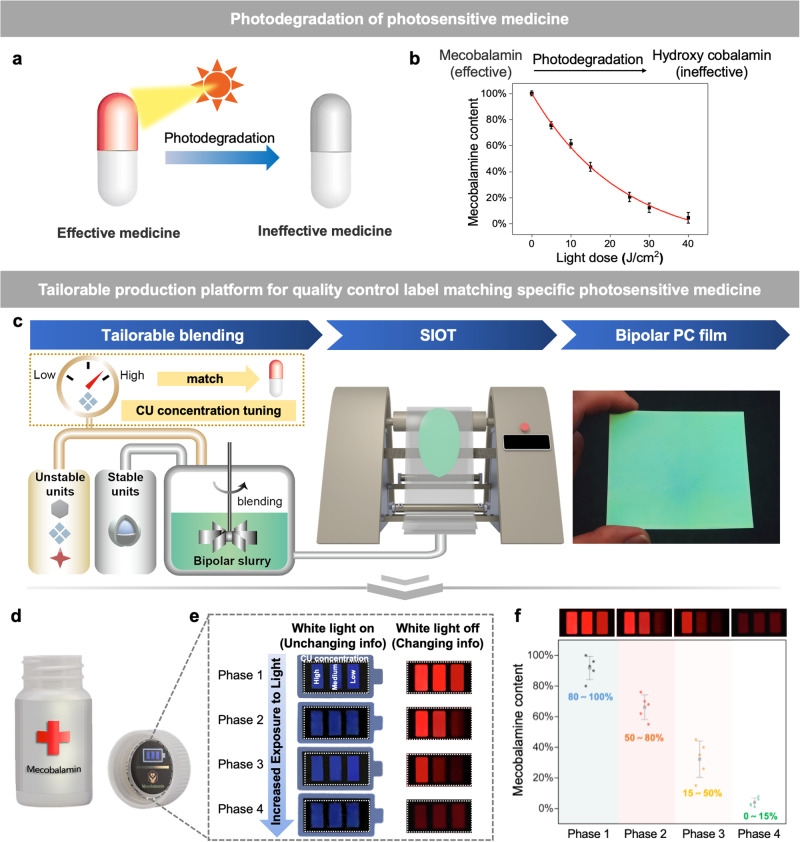
Fig. 4. Tailorable quality control label for photosensitive medicines based on the bipolar information indication system enabled by SIOT.
a Schematic diagram of photodegradation of photosensitive medicine (red pill refers to effective medicine while gray pill ineffective after exposed to excessive light). b Photodegradation curve of mecobalamin (a photosensitive medicine) under white light exposure. Error bars represent mean ± standard deviations. n = 5. c Mass production of bipolar PC films tailored to specific photosensitive medicine enabled by SIOT in the laboratory. In the left panel, low refers to CU concentration at 4 mM and high 12 mM. In the center panel, the machine can shear the disordered bipolar slurry prepared from left panel and form ordered bipolar PC films. The right panel shows the reflection color of the prepared PC film on a black background. d Schematic of a quality control label placed on photosensitive medicine. e Pictures of quality control label storing unchanging and changing information of the photosensitive medicine under different lighting conditions. (The CU concentration in the label from left to right, High: 12 mM, Medium: 8 mM, Low: 4 mM). f Remaining mecobalamin content in different phases of the quality control label. Error bars represent mean ± standard deviations. n = 5.