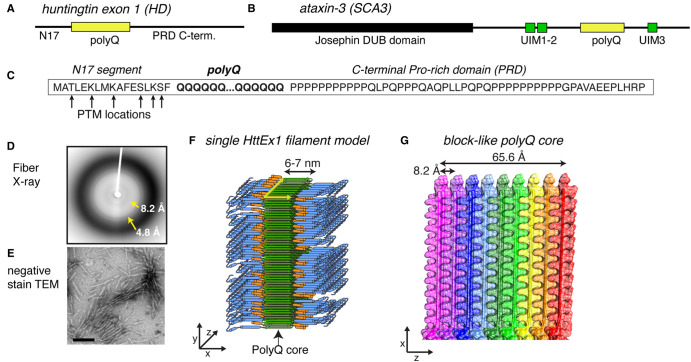
Figure 1. Summary of HttEx1 polyQ protein structures.
(A,B) Domain architecture of protein (fragments) from the most common polyQ diseases: HttEx1 from Huntington's disease (HD) and ataxin-3 from spinocerebellar ataxia 3 (SCA3). The polyQ segment is shown in yellow, alongside neighboring flanking domains with and without folded domains. HttEx1 is disordered in its monomeric state. (C) Sequence of HttEx1, with the location of prominent post-translational modifications indicated. The three key parts of HttEx1 are marked: N17, polyQ, and PRD. (D,E) X-ray fiber diffraction and negative stain transmission EM of aggregated polyQ/HttEx1. Adapted with permission from Hoop et al. [28]. (F) Structural model of aggregated HttEx1 from ssNMR and other techniques. (G) The polyQ core showing the block-like architecture that buries most of the polyQ segments. (F,G) Adapted with permission from Boatz et al. [33], under its CC-BY license.