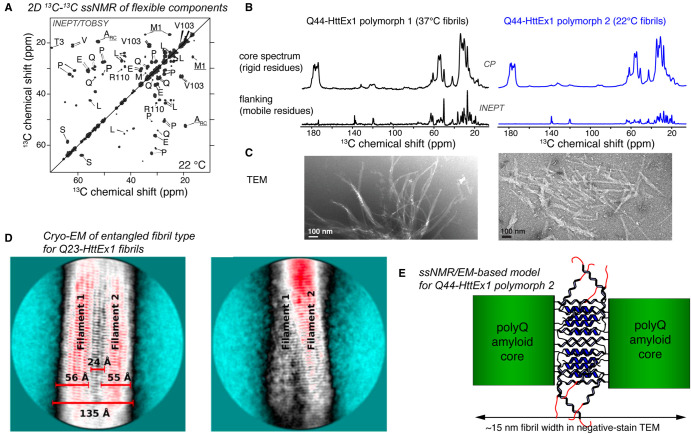
Figure 3. Studies of the HttEx1 flanking domains and their interactions.
(A) 2D ssNMR spectrum obtained by scalar-recoupling-based ssNMR spectroscopy, showing the flexible flanking domains of HttEx1 fibrils. (B) Comparison of 1D 13C traditional (top; CP) and scalar (bottom; INEPT) ssNMR spectra for different HttEx1 polymorphs, revealing primarily differences in flanking domain motion in the bottom spectra. (C) Negative-stain TEM of the same Q44-HttEx1 fibril types, which differed in their fibril widths. (D) Cryo-EM data on a sub-class of Q23-HttEx1 fibrils, showing an architecture mediated by flanking domain interactions. The two images represent different views on the same fibril type. (E) Model of flanking domain entanglement in protofilament interactions in wider HttEx1 fibrils (e.g. type 2 shown in B,C), deduced from combined analysis of negative stain TEM and ssNMR data. (A–C,E) Adapted with permission from Lin et al. [32], under the CC license. Panel (D) Reprinted with permission from Nazarov et al. [20] Copyright 2022 American Chemical Society.