Figure 2.
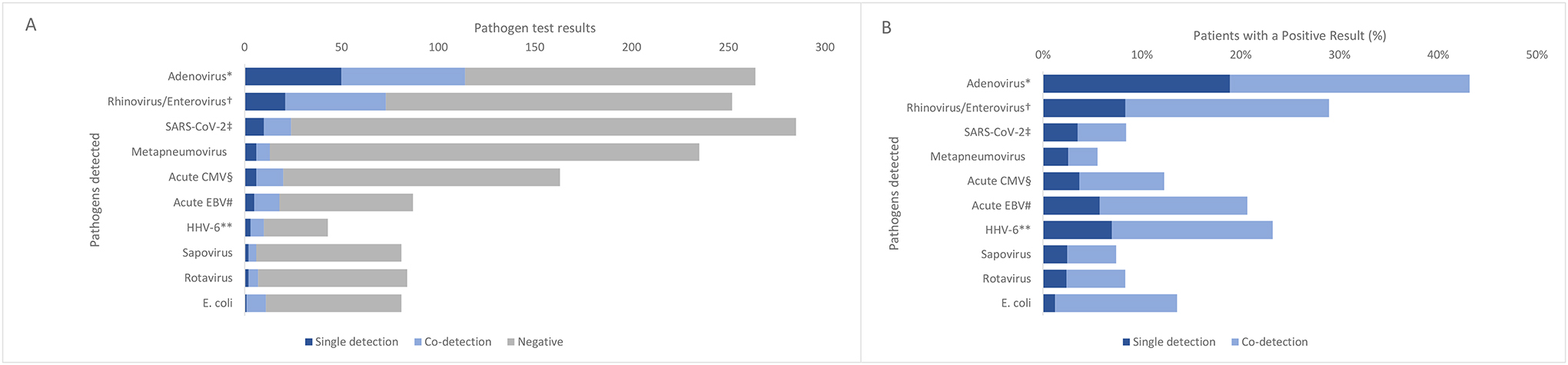
Absolute numbers (A) of single detection, co-detection, and negative test results for pathogens tested for and proportions (B) of patients with a positive single detection or co-detection result, among patients without significant underlying conditions (N=318).
Abbreviations: EBV=Epstein-Barr Virus; HHV=human herpes virus.
* Among any specimen type
† Combines testing for rhinovirus/enterovirus via multiplex panel as well as testing via enterovirus specific NAAT
‡ Current SARS-CoV-2 infection defined as a positive PCR or antigen test within 14 days of hepatitis onset.
§ Acute CMV based on clinical diagnosis, verified by PCR IgM result.
# Acute EBV defined as positive EBV viral capsid antigen (VCA) immunoglobulin M (IgM) or early antigen (EA) immunoglobulin G (IgG) test result, or diagnosis of primary EBV in the medical chart.
** HHV-6 defined as positive PCR or IgM; IgG antibody results were not included.
