Abstract
Productivity loss because of chronic pain in the working age population is a widespread concern internationally. Interventions for chronic pain in working age adults might be expected to achieve enhanced productivity in terms of reduced costs of workers' compensation insurance, reduced disability support, and improved rates of return to work for injured workers. This would require the use of measures of productivity in the evaluation of chronic pain management interventions. The aim of this review was to identify and interpret the productivity outcomes of randomised controlled trials reported by studies that conducted economic evaluations (eg, cost-effectiveness and cost-utility) of chronic pain management interventions in the working age population published from database inception to March 2023. Econlit, Embase, and Pubmed electronic databases were searched, yielding 12 studies that met the selection criteria. All 12 studies used absenteeism to measure productivity, translating return to work measures into indirect costs. Only one study included return to work as a primary outcome. Ten studies found no statistically significant improvements in productivity-related costs. Despite evidence for reduced pain-related disability after pain management interventions, this review suggests that the use of measures for assessing productivity gains is lacking. Including such measures would greatly assist administrators and payers when considering the broader societal benefits of such interventions.
Keywords: Pain, Chronic pain, Economic evaluation, Cost-effectiveness, Productivity, Return to work, Indirect costs, Productivity loss
1. Introduction
The Global Burden of Diseases, Injuries, and Risk Factors Study 2017 found that chronic pain was the leading cause of years lived with disability (YLD) rates across the world.17 Chronic pain is defined as pain lasting for at least 3 months.43 Chronic pain patients often need to give up work, hobbies, sport, and household chores.29 Chronic pain is associated with substantial economic burden due to healthcare resources used, productivity losses, and lower quality of life, including anxiety and depression.19,30,35 Sick-listed employees may find it difficult or impossible to return to the workplace because of the combined challenges of managing their pain and poor environmental support.30,49 Estimates of the prevalence of chronic pain are variable, depending on which definitions and methods are being used, and range from approximately 20% in Australia5 28.4% adults in the United States11 to up to 51.3% adults in the United Kingdom.14
Productivity costs occur when the productivity of individuals is affected by illness, treatment, disability, or premature death.19 Chronic pain management interventions aimed at functional improvements in working age patients have the potential to produce substantial societal gains because of improved productivity. Economic evaluations of an intervention can aid decision makers in the allocation of limited healthcare resources (a healthcare perspective) and help determine the benefit to society as a whole (the societal perspective). The relevance of including productivity loss (costs) or gain (benefits) in these evaluations is increasingly recognized, and therefore, studies quantifying productivity outcomes (costs and/or benefits) are needed.
In Australia, the cost of productivity losses associated with chronic pain was estimated to be AU$48.3 billion in 2018,12 and studies have indicated that older working age people with chronic pain have an increased risk of falling into income poverty.35 In the United States, Gaskin and Richard estimated that the annual cost of lost productivity because of pain (in 2010 dollars) was between US $299 and US $335 billion.16 In Chile, musculoskeletal chronic pain was estimated to cost 0.417% of the national gross domestic product (GDP) with more than US $19 million estimated to be due to productivity losses.48 Some groups of people with chronic pain seem to fare worse than others. For example, patients with chronic neuropathic pain appear generally in poorer health and to have higher costs of health care resource utilization and lower productivity than chronic pain patients with nonneuropathic pain.3,34
Because of the prevalence and generally poor outcomes of treatments for chronic pain, a wide variety of pain management approaches have been developed, along with guidelines for treatment.6 Not surprisingly, these come with a cost. Although several cost-effectiveness studies of chronic pain management interventions have been reported,7,33 a comprehensive review of the productivity outcomes of such interventions has not yet been conducted. As such, the aim of this review was to answer 2 questions:
(1) What productivity measures have been used in economic evaluations completed alongside randomised controlled trials (RCTs) of chronic pain management intervention in the working age population?
(2) What was the effect on productivity outcomes of those interventions?
2. Materials and methods
The systematic review followed Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement guidelines.28 The protocol for this study was published previously by these authors.8
2.1. Eligibility criteria
We determined study eligibility using the Population, Intervention, Comparison, Outcome framework27 and summarised as follows:
2.1.1. Study types
English-language economic evaluations (cost-effectiveness and cost-utility studies) of chronic pain management interventions arising from RCTs.
2.1.2. Participants
Working-age adults (18-65 years) experiencing musculoskeletal and/or neuropathic pain.
2.1.3. Interventions
Any nonpharmaceutical and nonsurgical interventions and any control.
2.1.4. Outcomes
Any reported productivity outcomes such as return to work or reduced sick leave.
Studies involving working-age adults with other conditions (such as pain related to pregnancy, cancer) were excluded. Grey literature and conference proceedings were also excluded.
Studies were grouped by type of study (intervention vs usual care and intervention vs alternate intervention) and productivity outcome for synthesis.
2.2. Identification and selection of studies
Four investigators (A.R.C., P.L.G., D.S. and M.N.) determined and used the following search strategy ((chronic) AND ((neck pain) OR (shoulder pain) OR (arm pain) OR (leg pain) OR (back pain) OR (neuropathic pain))) AND ((cost benefit) OR (cost effectiveness) OR (cost utility) OR (economic evaluation)). The electronic databases Econlit, Embase, and Pubmed were searched for relevant studies published from inception to March 2023. Abstracts and titles of the studies identified through the database search were screened independently by 2 investigators (A.R.C. and P.L.G.) to identify full-text English-language RCTs, including productivity outcomes for detailed review. Differences were resolved by consensus. Studies were included if they met the terms of the inclusion criteria.
2.3. Data extraction
Data extraction was performed using a standardized form. A.R.C. extracted study characteristics, including year of publication, country, settings, intervention(s), measures of clinical and healthcare utilization and follow-up and measures of productivity. These extracted data were checked and verified by 2 coauthors (D.S. and D.C.). Differences were resolved by consensus. Cost-effectiveness results were extracted from measures of both effectiveness (efficacy) and economic costs by A.R.C. and D.C. Measures of productivity, including the return to work, human capital approach (HCA), and/or friction cost methods (FCM), were extracted. The HCA measures the future monetary value of lost productivity such as sick leave or absenteeism at paid work because of illness or disability.23 The FCM restricts costs of productivity loss to the friction period—the period it takes to replace a worker because of illness, although internal resource reserves are taking up the work of a missing employee and the duration and the costs of hiring and training new workers taking into account the degree of scarcity of labour in the economy.22,46 Other cost-effectiveness measures extracted for this study were derived from health outcomes, standardized health-related quality of life measures, and measures to evaluate psychological components of chronic pain conditions and disability associated with the conditions.
2.4. Assessment of the health economic evaluation reporting standards and risk of bias
Data for the assessment of the economic evaluation reporting standards were extracted using the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) 2022 statement. This statement provides decision makers and researchers with guidance and a checklist to improve reporting.20 Key data extracted included the population of interest, perspective, comparators, time horizon, discounting, outcome measures, including efficacy, quality of life, and productivity measures, incremental costs and outcomes, analytical methods supporting the evaluation, measures of uncertainty, reporting style, findings of the study, sources of funding, and conflicts of interest. A.R.C. and D.S. jointly assessed the included studies. P.L.G. resolved disagreements where necessary.
Risk of bias was assessed independently by 2 authors (A.R.C. and P.L.G.) using the revised Cochrane risk of bias tool for randomized trials.40 This tool explicitly assesses the risk of bias associated with the randomization process, deviation from the intended interventions, missing outcomes, measurement of the outcomes, and selection of the reported results. Overall, where studies had one or more domains in which there were some concerns or high concerns about the risk of bias, these studies were categorised as at high risk of bias using the Cochrane tool. Differences were resolved by consensus.
2.5. Effect measures and synthesis
The primary outcome of interest was the difference in productivity measures between intervention arms. Because of anticipated variability in trials designs, interventions applied, cohorts studied, and definitions and methods of collecting productivity measures, a narrative synthesis was planned.
3. Results
3.1. Flow of studies through the review
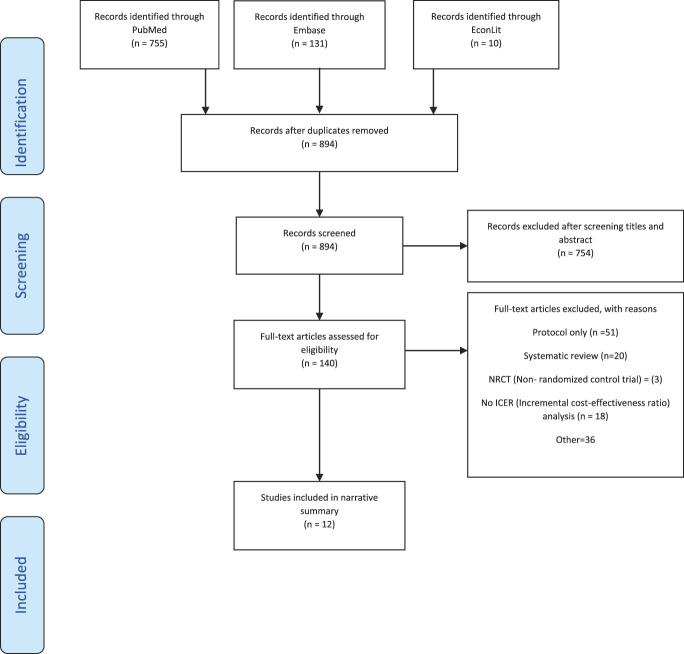
A total of 896 studies were identified through the initial search. After removing 2 duplicates, 894 titles and abstracts were screened for inclusion. Of these, 140 full-text articles were assessed for eligibility with 12 RCTs included in the narrative synthesis. Figure 1 shows the flow diagram.
Figure 1.
PRISMA flowchart of the study selection process. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
3.2. Characteristics of studies included
The included study characteristics are summarised in Table 1. The included studies were all from Europe and the United Kingdom, 91.7% (11/12) were on low back pain, 1 study was on whiplash or neck disorder,25 and 1 study included patients with unspecific chronic pain.21 Among the included studies, 58.3% (7/12) involved intervention vs usual care and the rest (41.7%) comprised comparison of 2 or more interventions. All studies had similar cohorts in terms of age (working age population) and their chronic pain duration (typical duration >3 months).
Table 1.
Characteristics of included studies.
| Authors, y (additional supporting papers used for data extraction such as study protocols etc) | Setting (outpatient, primary, secondary etc) | Interventions | Target population/sample size | Details of the economic evaluation | Measures of clinical and healthcare utilization and informal care cost outcomes and follow-up | Measures of productivity |
|---|---|---|---|---|---|---|
| Apeldoorn et al. 20122 Apeldoorn et al. 20101 | Primary and secondary care | Intervention 1: treatment according to a classification system (direction-specific exercises, spinal manipulation or stabilization exercises) Intervention2: usual physical therapy according to Dutch low back pain (LBP) guidelines |
Age: 18-65 y F: 56.95% Pain type: subacute and chronic low back pain (CLBP) Pain duration: >6 wk Total sample size: 156 |
Country: Netherlands Currency: Euro Time horizon: 1 year Perspective: societal Type of economic evaluation: CEA and CUA Reference year: 2009 Discounting: not applied as 1 y time horizon |
Health outcome measure: Global perceived effect measured by self-assessed 7 points Likert scale Pain intensity using an 11-point numerical rating scale (NRS) Health-related quality of life using EQ-5D Quality-adjusted life years (QALYs) measured by multiplying utility of a health state by the time spent in this health state Cost measures: Direct cost: Healthcare utilization (HCU) cost: Primary care cost Secondary care cost Nonhealthcare cost: Informal care (per hour) Paid home help (per hour) Follow-up: 8, 26,39, and 52 wk |
Reported as Indirect cost: Cost of absenteeism by mean productivity cost (GBP) per hour using Friction Cost Method (FCM) and Disease Questionnaire (PRODISQ) (sensitivity analysis was conducted using human capital approach (HCA)) |
| Chuang et al. 20129 Cox et al. 201010 |
Primary and secondary care | Intervention 1: yoga+ usual care Intervention 2: usual care (any ongoing treatment) |
Age: 18-65 y F:N/A Pain type: CLBP Pain duration: 18 mo Total sample size: 313 |
Country: UK Currency: GBP Time horizon: 1 y Perspective: National Health Services (NHS) and Societal Type of economic evaluation: CEA and CUA Reference year: 2008-2009 Discounting: N/A |
Health outcome measure: Roland–Morris disability questionnaire (RMDQ) to measure back function EQ-5D to measure QALYs HCU: Primary care cost Secondary care cost Private care cost Nonhealthcare cost: Equipment purchase cost Follow-up: 3, 6, and 12 mo |
Reported as Other costs: Cost of absenteeism by number of days off work in terms of GBP (national income per day) |
| Goossens et al. 201518 Leeuw et al. 200826 |
Multicentre (Hospital) settings | Intervention 1: exposure in vivo (EXP) (CBT, educational sessions) Intervention2: graded activity—CBT one session |
Age: 18-65 y F: 50% Pain type: LBP Pain duration: ≥3 mo Total sample size:62 |
Country: Netherlands Currency: Euro Time horizon: 15 mo Perspective: type of economic evaluation: Reference year: 2014 Discounting: N/A |
Health outcome measure: 36-item Short-Form Health Survey (SF-36) Quebec back pain disability scale Cost measures: Healthcare costs Intervention cost Patient and family costs Productivity loss Follow-up: 6,12 mo |
Cost of absenteeism was measured by the number of days off for back pain multiplied by cost per day using HCA Also estimated using the FCM in a sensitivity analysis |
| Kemani et al. 201521 | Hospital services | Intervention 1: acceptance and commitment therapy Intervention2: applied relaxation |
Age: 18-65 y F:73% Pain type: chronic unspecific pain Pain duration: ≥6 mo Total sample size:60 |
Country: Sweden Currency: USD Time horizon: 6 mo Perspective: societal Type of economic evaluation: CEA Reference year: 2013 Discounting: N/A |
Health outcome measure: The Pain Disability Index (PDI) (0-10 scale)to assess the disabling effects of chronic pain on daily activities The Hospital Anxiety and Depression Scale (HADS) was used to assess anxiety and depression Acceptance of pain using the Chronic Pain Acceptance Questionnaire (CPAQ) Short Form-12 health survey (SF-12) to assess health-related quality of life Cost measures: direct healthcare utilization cost Direct nonmedical cost Productivity loss Follow-up: posttreatment, 3 and 6 mo |
Productivity losses were estimated using the HCA, ie, monetary losses associated with work loss and work cutback were based on the average gross earning in Sweden for the duration of the reported number of days off |
| Lambeek et al. 201024 | Primary and secondary care | Intervention 1: integrated care Intervention2: usual care with advice (following the Dutch physiotherapy guideline) |
Age:18-65 y F: 63% Pain type: CLBP Pain duration:>3 mo Total sample size: 134 |
Country: Netherlands Currency: Time horizon: Perspective: societal Type of economic evaluation: CBA,CEA,CUA Reference year: 2007 Discounting: N/A |
Health outcome measure: Duration until sustainable return to work QALYs using the Euro- Qol Cost measures: Direct healthcare cost Nondirect healthcare cost Productivity loss Follow-up: 12 mo |
HCA to calculate the costs of productivity loss as a result of days off (work hours multiplied by per hour cost of productivity loss) |
| Landén Ludvigsson et al. 201725 | Multicentre | Intervention 1: physiotherapist-led neck-specific exercise (NSE) Intervention2: NSE with a behavioural approach (NSEB) Intervention 3: prescription of physical activity |
Age: 18-63 y F:65% Pain type: chronic whiplash-associated disorders (WAD) Pain duration: 6-36 mo Total sample size:216 |
Country: Sweden Currency: USD Time horizon: 12 mo Perspective: societal Type of economic evaluation: CUA Reference year: 2016 Discounting: N/A |
Health outcome measure: The neck disability index (NDI) EQ-5D SF-6D Cost measures: Healthcare utilization cost Intervention cost Productivity loss Follow-up: 3, 6, and 12 mo |
Productivity loss was calculated using the HCA including gross salary plus taxes |
| Niemisto et al. 200531 | Intervention 1: manipulative-treatment group Intervention2: physician's consultation group |
Age: 24-48 y F: 55% Pain type: CLBP Pain duration:>3 mo Total sample size: 204 |
Country: Finland Currency: USD Time horizon: 12 mo Perspective: societal Type of economic evaluation: CEA Reference year: 2002 Discounting: N/A |
Health outcome measure: A visual analogue scale (VAS; from 0 to 100) The Oswestry Low Back Pain Disability Questionnaire (ODI; from 0 to 100) Health-related quality of life (HRQoL) (15D) Cost measures: Health care utilization cost Productivity loss Follow-up: 5 and 12 mo; 2 y |
Productivity costs because of absence from work. Productivity costs were estimated by the average 2000-y wage level in Finland | |
| Schweikert et al. 200636 | Intervention 1: usual care + cognitive behavioural pain management program Intervention2: usual care (including physiotherapy, massage, seminars, and exercise) |
Age: ≥18 y F:17.2% Pain type: CLBP Pain duration: >6 mo Total sample size:409 |
Country: Germany Currency: Euro Time horizon: 6 mo Perspective: societal Type of economic evaluation: CEA, CUA Reference year: 2001 Discounting: N/A |
Health outcome measure: VAS Euro-QoL Cost measures: Healthcare utilization cost Nonmedical cost Productivity loss Follow-up: 1 and 6 mo |
Productivity costs were estimated by age- and sex-adjusted average labor costs incorporating salaries and social insurance premiums paid by employers and employees because of days off at work | |
| Smeets et al. 200939 | Outpatient rehabilitation centres | Intervention 1: active physical training (APT) Intervention 2: behavioural therapy (GAP) Intervention 3: APT + GAP (combined training) |
Age:18-65 y F: 45% Pain type: LBP Pain duration: ≥3 mo Total sample size: 160 |
Country: Netherlands Currency: Euro Time horizon: 12 mo Perspective: societal Type of economic evaluation: CEA, CUA Reference year: 2003 Discounting: N/A |
Health outcome measure: RMDQ Euro-QoL Cost measures: Direct healthcare cost Nondirect healthcare cost Productivity loss Follow-up: 6, 12 mo |
Absenteeism from paid work was calculated according to the HCA |
| Thomas et al. 200542 | Primary and secondary care | Intervention 1: acupuncture Intervention2: usual care (pragmatic GP management, with no restrictions on the care they received) |
Age:18–65 y F:60.2% Pain type: nonspecific LBP Pain duration: 4–52 wk Total sample size: 241 |
Country: UK Currency: GBP Time horizon: 24 mo Perspective: societal Type of economic evaluation: CEA and CUA Reference year: FY:2001-02 Discounting: 3.5% |
Health outcome measure: Short form 36 (SF-36) bodily pain dimension (range 0-100 points) EuroQoL 5 dimensions (EQ-5D) McGill present pain index (PPI) Oswestry Pain Disability Index (ODI) Cost measures: Healthcare utilization cost Productivity loss Follow-up: 3, 12, and 24 mo |
Employment status and time lost from work because of lower back pain in terms of GBP using age- and gender-adjusted daily wage |
| Van der Roer 200847 | Primary care | Intervention 1: intensive group training protocol Intervention2: usual care (physiotherapy) |
Age:18-65 y F:N/R Pain type: Non-specific CLBP Pain duration: >12 wk Total sample size: 114 |
Country: Netherlands Currency: Euro Time horizon: 12 mo Perspective: societal Type of economic evaluation: CEA, CUA Reference year: 2004 Discounting: N/A |
Health outcome measure: RMDQ Pain intensity measure General perceived effects measure scale Euro-QoL-5D Cost measures: Direct healthcare utilization cost Indirect healthcare utilization cost Productivity loss Follow-up: 6, 13, 26, and 52 wk |
Absenteeism from paid work using HCA |
| Werner et al. 201652 | Primary settings | Intervention 1: cognitive-based education program (CBEP) Intervention2: usual care (provided by general practitioners [GP] and physiotherapists [PT]) |
Age: 20-55 y F: 58.5% Pain type: unspecific LBP Pain duration: 4-12 mo Total sample size:216 |
Country: Norway Currency: USD Time horizon: 12 mo Perspective: societal Type of economic evaluation: CEA,CUA Reference year: 2012 Discounting: N/A |
Health outcome measure: RMDQ EQ-5D Cost measures: Healthcare utilization cost Productivity loss Follow-up: 4 wk, 3, 4, 6, and 12 mo |
Absenteeism costs were estimated by multiplying the number of days absent from work by the average wage rate |
It was necessary to consult other publications1,10,26 on these studies to determine some of the information.
3.2.1. Health outcome measures
The included studies used various health outcome measures, including numerical or Likert rating scales for pain intensity (4 studies)2,31,36,47 or pain index questionnaires (1 study42) and functional status (9 studies)9,18,21,24,25,36,42,47,52 and measures of anxiety and depression (1 study21) and pain acceptance (1 study21).
3.2.2. Quality-adjusted life years
Quality-adjusted life years (QALYs) were measured by all studies. One study measured QALYs by multiplying utility of a health state by the time spent in this health state,18 9 studies evaluated health-related quality of life using the EQ-5D,1,9,24,25,32,36,39,42,47,52 36-item Short-Form Health Survey (SF-36)50 (2 studies),18,42 Short Form-12 Health Survey (SF-12)51 (1 study),21 SF-6D (2 studies),25,53 and health-related quality of life (HRQoL) (15D)38 (1 study).18
3.2.3. Economic evaluation
All 12 included studies measured costs from a societal perspective. The study of Chuang et al. also measured costs from a healthcare perspective.9 Cost-effectiveness of the intervention of interest was established in 6 studies.9,18,24,25,31,36 The study conducted by Kemani et al.21 established cost-effectiveness of the intervention at posttreatment and in 3 months but not at 6 months; however, differences in costs and outcomes were not statistically significant except for the differences in the productivity outcome.
3.2.4. Productivity outcome
All of the included studies calculated and reported productivity loss using absenteeism from paid work translating return to work measures into indirect costs (cost savings) in monetary terms (indicated by an amount of money such as UK pound sterling, Euro etc). All but 1 study2 used the HCA to calculate absenteeism from paid work with the study by Goossens et al.18 and also conducting a sensitivity analysis by applying the FCM. The remaining study, Apeldoorn et al.,2 calculated the cost of absenteeism using the FCM and conducted a sensitivity analysis by applying the HCA. The study of Lambeek et al.24 was the only one to capture productivity gain by including return to work as their primary outcome.
3.3. Assessment of the health economic evaluation reporting standards
Results of the CHEERS assessment, presented in Figure 2, shows the proportion of RCTs for which a yes (Y), not reported (N), or not applicable (NA) response was obtained for each CHEERS checklist item. All studies addressed items 1 to 3, 5 to 9, 11 to 15, 19, 20, 23, 26, and 27 (the list of items and detailed interpretations is shown in Husereau et al).20 None of the studies used modelling (items 16) or engaged with noninvestigator stakeholders (items 21 and 25) and only 1 study (8%)42 used discounting (item 10). Only 2 studies2,9 validated data for statistical analysis (item 17), and 4 studies2,9,31,42 described subgroup results (item 18). In 2 studies, conflicts of interest did not appear to be reported36,39 (item 28) and in 5 studies,21,25,36,39,42 separate publication of a health economic analysis plan was not reported (item 4). The results implied reasonable methodological quality overall.
Figure 2.
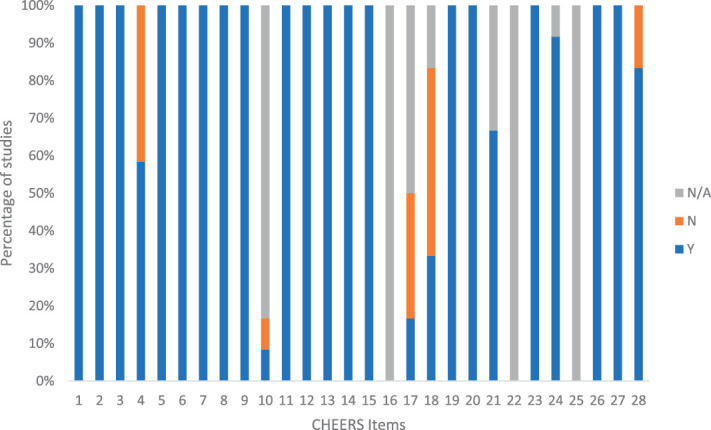
Proportion of yes, no (not reported), and not applicable responses to each Consolidated Health Economic Evaluation Reporting Standards (CHEERS) checklist item by the 12 included studies. Responses to each checklist item was recorded as N/A, not applicable; N, not reported; Y, reported.
3.4. Risk of bias
Responses to the risk of bias assessment were similar across all included studies (Fig. 3). Most studies were considered to have a “low risk of bias” for most domains; 91.7% of studies had low risk of bias for “randomisation process” and “deviations from intended interventions,” 66.7% for “missing outcome data,” and 50% for “selection of the reported result.” For the domain “measurement of the outcome,” most studies were deemed to have a high risk of bias (91.7%) as outcomes were self-reported. All but 2 studies2,52 were assessed to have a high risk of bias with this overall result arising from having one or more domains considered high risk.
Figure 3.
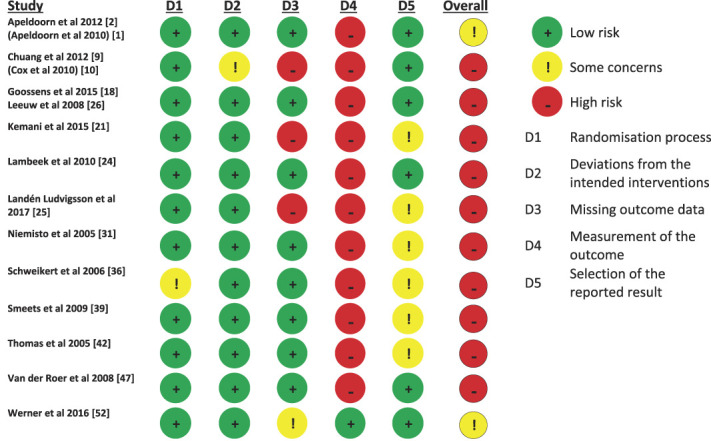
Cochrane Risk of Bias (RoB 2 tool) summary.
3.5. Summary of productivity outcomes of chronic pain management and cost-effectiveness of the included studies from chronic pain management interventions
Summaries of the productivity and other cost-effectiveness outcomes for each study are shown in Table 2.
Table 2.
Results of included studies.
| Studies | Costs, mean (SD) Mean difference (95% CI) (Societal perspective) |
Costs of productivity, mean (SD) Mean difference (95% CI) |
Effects, mean (SD) Mean difference (95% CI) |
Reported ICER | Author's conclusion: cost-effective? | Mean (SD) number of sick leave at follow-up |
|---|---|---|---|---|---|---|
| Apeldoorn et al. 20122 Apeldoorn et al. 20101 |
Intervention 1: €2287 (482) Intervention 2: €2020 (331) Mean difference (95% CI) = €266 (−720; 1612) |
Intervention 1: €1575 (378) Intervention 2: € 1208 (289) Mean difference (95% CI) = €367 (−423, 1545) |
Intervention 1: GPE = 0.68 (0.06) ODI improvement = −8.2 (1.7) NRS improvement = −2.83 (0.40) QALYs gained = 0.82 (0.02) Intervention 2: GPE = 0.47 (0.06) ODI improvement = −7.8 (1.7) NRS improvement = −2.69 (0.35) QALYs gained = 0.80 (0.02) Mean (SD) difference (Int 1 − Int 2): GPE = 0.20 (0.04, 0.37) ODI improvement = 0.5 (−4.4, 5.4) NRS improvement = 0.13 (−0.86, 1.12) QALYs gained = 0.02 (−0.03, 0.08) |
ICER based on GPE = €1299 ICUR based on QALYs = €10,543 cost per QALYs gained |
Intervention 1 not cost-effective compared with Intervention 2 (Outcome and cost differences were not statistically significant) Authors do not recommend widespread approach | Intervention 1: 12.6 d (30.4) Intervention 2: 14.0 d (47.2) |
| Chuang et al. 20129 Cox et al. 201010 |
Intervention 1: £1502.1 (2550.4) Intervention 2: £2319.2 (3188.1) Mean difference (95% CI) = −£ 667.48 (−1492.9 to 157.9) |
Intervention 1: £374.2 (1142.5) Intervention 2: £ 1201.8 (2550) Mean difference = −£827.6 |
Intervention 1: QALYs gained = 0.778 (0.143) Intervention 2: QALYs gained = 0.725 (0.172) Mean difference (95% CI): QALYs gained = 0.054 (0.021-0.088) |
NHS perspective, ICUR based on QALYs = £13,606 cost per QALYs gained Societal perspective, ICUR based on QALYs = £20,000 cost per QALYs gained |
Intervention 1 was cost-effective compared with intervention 2. Cost of productivity lost = 1201.8 (UC) vs 374.2 (yoga) | Intervention 1: 3.83 d (SD: 11.68) Intervention 2: 12.29 d (SD: 26.07) |
| Goossens et al. 201518 Leeuw et al. 200826 |
Intervention 1: €13,477.71 (2450.28) Intervention2: €10,843.50 (1747.89) Mean difference (95% CI): −€2643 (−8535 to 3058) |
Intervention 1: €1126.98 (355.07) Intervention2: €754.83 (255.86) Mean difference (95% CI): €372.15 (−4987 to 1908) |
Intervention 1: QPBDS: 40.42 (22.34) SF36:0.68 (0.14) QALY:0.82 (0.12) Intervention 2: QPBDS:38.19 (20.84) SF36:0.66 (0.14) QALY:0.83 (0.13) Mean difference (95% CI): QPBDS: −2.23 (−13.20 to 8.75) SF36: −0.15 (−0.08 to 0.05) QALY: 0.01 (−0.6 to 0.07) |
Based on QPBDS, €16,000 for an additional improvement in QPBDS Based on QALYs, 0.01 for an additional QALY |
Intervention 2 cost-effective compared with intervention 1 but differences were not significant | Intervention 1: 191.39 h (SD 362.60 h) Intervention2: 291.69 h (SD 501.99 h) |
| Kemani et al. 201521 | Intervention 1: $7836 (5676) Intervention 2: $5694 (4713) |
Intervention 1: $5406 (4258) Intervention 2: $3664 (3650) |
6-mo follow-up: Intervention 1: PDI: 31.2 (19.0) Pain:4.4 (1.3) SF12M:39.3 (10.8) SF12P:39.3 (10.2) HADS-a:9.1 (5.1) HADS-d:8.4 (5.6) CPAQ: 63.4 (21.2) Intervention2: PDI: 34.0 (16.2) Pain: 4.1 (1.5) SF12M: 38.8 (13.8) SF12P: 32.3 (9.8) HADS-a: 9.2 (5.1) HADS-d: 8.4 (5.5) CPAQ: 50.2 (21.9) |
At 3 mo follow-up: −648, indicating that each incremental improvement on the PDI for participants in ACT relative to AR generated a societal earning of $648 |
Intervention 1 was more cost-effective compared with Intervention 2 at 3-mo follow-up but not at 6-mo follow-up. Differences in indirect costs were significant (as determined by sign tests) | Intervention 1: 67 h (SD 246 h) Intervention2: applied relaxation (AR)= 1002 h (SD 2081 h) |
| Lambeek et al. 201024 | Intervention 1: £13,165 (SD £13,600) Intervention 2: £18,475 (SD £13,616) Mean difference (95% CI): −£5310 (−10,042 to −391) |
Intervention 1: £11,686 (SD £12,553) Intervention2: £17,213 (SD £13,416) Mean difference (95% CI): −£5527 (−10160 to −740) |
Intervention 1: Days until sustainable return to work: 129 (117) QALYs: 0.74 (0.19) Intervention 2: Days until sustainable return to work: 197 (129) QALYs: 0.65 (0.21) Mean difference (95% CI): Days until sustainable return to work: −68 (−110 to −26) QALYs: 0.09 (0.01-0.16) |
Days until sustainable return to work: £ −3 QALYs: £A61,000 cost per QALYs gained |
Intervention 1 would significantly reduce societal costs, increase effectiveness of care, improve quality of life, and improve function on a broad scale compared with intervention 2 | Intervention 1: 88.5 number of net days (SD 95.5 number of net days) Intervention2: 130.4 number of net days (SD 102.7 number of net days) Mean difference of net sick leave at 12 mo follow-up −68 d |
| Landén Ludvigsson et al. 201725 | Intervention 1: $2976 (SD $7650) Intervention2: $6810 (SD $13,453) Intervention 3: $5.349 (SD $10,429) |
Intervention 1: $2154 (SD $6963) Intervention2: $5556 (SD $13,058) Intervention 3: $4147 (SD $10,003) |
Intervention 1: Utility change score EQ-5D: 0.046 (0.208) NDI: 0.040 (0.10) SF-6D: 0.054 (0.113) QALY gained EQ-5D: 0.023 (0.103) NDI: 0.020 (0.050) SF-6D: 0.027 (0.056) Intervention 2 Utility change score EQ-5D: 0.106 (0.252) NDI: 0.055 (0.086) SF-6D: 0.028 (0.106) QALY gained EQ-5D: 0.053 (0.126) NDI: 0.028 (0.043) SF-6D: 0.014 (0.053) Intervention 3: Utility change score EQ-5D: −0.038 (0.282) NDI: −0.006 (0.080) SF-6D: 0.002 (0.112) QALY gained EQ-5D: −0.019 (0.141) NDI: −0.003 (0.040) SF-6D: 0.001 (0.060) |
Intervention 1 vs 2: Societal perspective: EQ5D: 127,800 (95% CI: 37,816-711,302) NDI: 14,400 (5039-74,484) Healthcare perspective: EQ5D: $479,250 (49,160-2,951,905) NDI: $54,000 (6550-309,197) |
Intervention 1 was cost-effective compared with intervention 2 and 3 | Intervention 1: 13.8 d (SD 43.7 d) Intervention2: 29.7 d (SD 74.9 d) Intervention 3: 21.6 d (SD 46.5 d) |
| Niemisto et al. 200531 | Intervention 1:$2262 (SD $3156) Intervention 2: $2280 (SD $5294) Mean difference =-$18 |
Intervention 1: $1632 (SD $2728) Intervention 2: $1970 (SD $5068) Mean difference = −$338 |
Intervention 1: VAS: 30.7 (24.4) The Oswestry Low Back Pain Disability Questionnaire = 12.0 (11.6) HRQoL(15D) = 0.91 (0.078) Intervention 2: VAS: 33.1 (24.9) The Oswestry Low Back Pain Disability Questionnaire = 14.0 (9.9) HRQoL(15D) = 0.91 (0.082) |
Intervention 1 vs Intervention 2: VAS: $512 (15,714) The Oswestry Low Back Pain Disability Questionnaire = $78 (20,818) |
Intervention 2 was cost-effective than intervention 1. Productivity costs were lower in the physician's consultation group compared with the manipulative treatment group | Intervention 1: 12.3 d (SD 20.5 d) Intervention2: 14.8 d (SD 38.0 d) |
| Schweikert et al. 200636 | Intervention 1: €8849.3 (SD €5820.0) Intervention 2: $10,519.9 (SD €8073.6) Mean difference: –$1670.6 (P-value for the diff −0.054) |
Intervention 1: €1441.1 (SD €3713.4) Intervention 2: €2192.1 (SD €4622.9) Mean difference: −€751 |
Incremental effects: Intervention 1: ∆ admission − discharge: EuroQoL: 9.6 (18.3) Functional capacity: 2.8 (12.3) Depression: −2.3 (4.7) Anxiety: −2.7 (6.9) Subjective back pain: −1.2 (1.2) ∆ 6-mo follow-up–discharge: EuroQoL: −2.4 (17.8) Intervention 2: ∆ Admission–discharge: EuroQoL: 9.3 (15.2) Functional capacity: 3.5 (13.4) Depression: −1.6(4.2) Anxiety: −2.3 (6.3) Subjective back pain: −1.2 (1.2) ∆ 6-mo follow-up–discharge EuroQoL: −4.5 (14.9) |
–€126,731 cost per QALY gained | Intervention 1 may be more cost saving than intervention 2 | Intervention 1: 11.4 d (SD 28.9 d) Intervention2: 16.8 d (SD 34.1 d) |
| Smeets et al. 200939 | Intervention 1: €20,015 (SD €19,675) Intervention 2: €14,794 (SD €17,209) Intervention 3: 19,559 (SD €14,708) Mean difference (95% CI): (3 vs 1): −€407 (−6987 to 5900) Mean difference (95% CI): (3 vs 2): −€4787 (984-10,540) |
Intervention 1: €16,153 (SD €18,748) Intervention 2: €11,816 (SD €15,804) Intervention 3: €14,987 (SD €1562) Mean difference (3 vs 1): −€1166 Mean difference (3 vs 2): €3171 |
RDQ Mean difference (3 vs 1): −1.23 (−3.01 to 0.55) Mean difference (3 vs 2): −1.27 (−2.96 to 0.42) QALY: Mean difference (3 vs 1): −0.014 (0.094-0.066) Mean difference (3 vs 2): −0.045 (−0.119 to 0.029) |
RDQ: Intervention 3 vs 1: APT 371 Intervention 3 vs 2: GAP −3759 QALY: Intervention 3 vs 1: APT 35,060 Intervention 3 vs 2: GAP −108,857 |
Intervention 3 was not more cost-effective than intervention 1 or 2 | Intervention 1: 906.94 h (SD 1052.64 h) Intervention 2: 663.44 h (SD 887.35 h) Intervention 3: 841.50 h (SD 922.84 h) |
| Thomas et al. 200542 | Intervention 1: £2135.39 (£3798.45) Intervention 2: £2469.09 (£3618.97) Mean difference (95% CI): –e333.70 (–31601.92 to £1179.81) |
Intervention 1: £1679.99 (£4812.54) Intervention 2: £2321.68 (£6011.38) Mean difference (95% CI): –e641.69 (–42130.62 to £1299.52) |
AUC using SF6D: Mean difference (95% CI): 0.027 (−0.056, 0.110) | £4241 (95% CI: £191 to £28,026) | Intervention 1 was significantly more effective with a higher cost (difference in societal costs were not statistically significant) | Intervention 1: 16.086 total days (0-24 mo) (SD 43.271 d) Intervention 2: Usual care = 20.13 total days (0-24 mo) (SD 53.739 d) |
| Van der Roer et al. 200847 | Intervention 1: €3891 (SD €7011) Intervention 2: €3658 (SD €5970) Mean difference (95% CI): €233 (185-2764) |
Intervention 1: € 2770 (SD €6643) Intervention 2: €2838 (SD €5814) Mean difference (95% CI): −€68 (−2504 to 2302) |
Mean difference (95% CI): Functional status (RDQ): 0.06 (−2.22 to 2.34) Pain intensity (PI-NRS): −1.02 (−2.14 to 0.09) Perceived recovery (GPE): 13% OR 1.71 (0.67-4.38) QALYNL (EQ-5D): 0.03 (−0.06-0.12) |
Functional status (RDQ): 16,349 Pain intensity (PI-NRS): −175 Perceived recovery (GPE): 1720 QALYNL (EQ-5D): 5141 |
No differences between the interventions | N/A |
| Werner et al. 201652 | Intervention 1: $15,362 (95% CI 9076-21,036) Intervention 2: $15,580 (95% CI 7634-24,427 (costs because of work loss) Mean difference: −$101 |
Intervention 1: $15,113 Intervention 2: $15,230 Mean difference: −$117 |
Intervention 1: RMDQ: 3.6 (4.2) Pain Intensity: 2.8 (2.5) E0Q5D: 0.8 (0.2) Intervention 2: RMDQ: 3.0 (3.4) Pain intensity: 2.6 (2.2) E0Q5D: 0.8 (0.2) Mean difference (95% CI): RMDQ: −0.42 (−1.27 to 0.42) Pain intensity: −0.27 (−1.02 to 0.48) E0Q5D: −0.007 (0.08-0.06) |
0.005 QALYs (CI 0.016-0.027) | No clinical or health economic benefits for intervention 1 | Mean difference in the number of sick days for total period: −2.47 (95% CI: −8.04 to 3.10) |
ACT, Acceptance and Commitment Therapy; AR, Applied Relaxation; CPAQ, Chronic Pain Acceptance Questionnaire; GPE, Global Perceived Effect; HADS, Hospital Anxiety and Depression Scale; ICER, Incremental Cost-effectiveness Ratio; NRS, numerical rating scale; ODI, Oswestry Pain Disability Index; PDI, Pain Disability Index; QALY, quality-adjusted life year.
3.6. Intervention vs usual care
Seven studies conducted an economic evaluation study comparing a chronic pain management intervention against usual care (UC).2,9,24,36,42,47,52 Usual care typically included advice and/or physical therapy and interventions varied broadly from acupuncture, spinal manipulation, or yoga training to physical therapy or behavioural multidisciplinary programs using combinations of cognitive behavioural therapy (CBT), physical activity, and/or physical therapy (Table 1). Differences in productivity loss were not statistically significant in 6 studies.2,9,36,42,47,52 Only the study by Lambeek et al. reported that productivity loss was significantly lower for the integrated care group compared with the UC group (P = 0.002).24 One study2 included both subacute and chronic pain patients and did not conduct separate analysis by pain subgroups; this study was included in this narrative review for completeness.
3.7. Intervention vs other intervention
Five studies conducted an economic evaluation study comparing 2 or more chronic pain management interventions.18,21,25,31,39 Interventions included relaxation, CBT, exercise, and spinal manipulation. All studies calculated productivity loss through absenteeism from paid work and applied HCA for all intervention arms. The study by Kemani et al.21 reported absenteeism in terms of work loss and work cutback and found a marginally statistically significant (P = 0.046) decrease in work cutback at the 3-month follow-up for the applied relaxation group compared with the acceptance and commitment therapy group, but this was not maintained at the 6-month follow-up. Differences in productivity loss were not statistically significant in the other studies.18,25,31,39
4. Discussion
This study is the first to comprehensively review productivity outcomes in cost-effectiveness studies of nonpharmaceutical-based chronic pain intervention in the working age population. Twelve studies were identified that met our inclusion criteria, 7 compared cost-effectiveness of chronic pain interventions vs usual care,2,9,24,36,42,47,52 and the rest compared 2 or more interventions.2,18,21,25,31,39 Methods of usual care and interventions varied widely between studies and limited the generalisability of the outcomes.
All but 221,24 of the studies found no statistically significant difference in productivity outcomes, that is, no evidence of a difference in the cost of absenteeism between the trial arms. This is not surprising because of the extensive variability in costs combined with relatively small sample sizes. Most studies lacked power to find significant productivity cost differences, but such comparisons were not the primary outcome of interest. It is also important to note that the costs of productivity losses may vary depending on the methods applied (ie, the friction cost method rather than the human capital approach).25 One of the studies included here2 used the FCM to calculate absenteeism from paid work and found, in a sensitivity analysis, different results using the HCA. However, another included study concluded that the costs of absenteeism did not change while applying the FCM and HCA.18
Of the 2 studies21,24 that did find a significant difference, the study by Lambeek et al.24 included duration until sustainable return to work as an outcome measure. This study suggested that integrated care had larger gains for patients with LBP, society, and employers as the intervention significantly improved patient's quality of life, thereby reducing social costs and patients' functional ability compared with those of usual care. The study by Schweikert et al.36 was aimed at patients' return to work. However, as return to work was not included as an outcome measure, it was not possible to evaluate this outcome. No other included studies had assessed return to work as a primary outcome but instead translated return to work measures into indirect costs (cost savings) by calculating and reporting productivity loss using absenteeism from paid work (6 studies).2,9,18,39,47,52
Pain interventions are likely to be more cost-effective if the participants in that intervention return to work.4 However, caution is needed in interpreting return to work as an outcome in isolation because it is possible that premature return to work may result in lower productivity and costs if the worker is still troubled by their pain, although they are back at work. This is known as “presenteeism” or working when unwell. There is evidence that if presenteeism is not managed well, the costs can be higher than those because of absenteeism.44 Previous studies also suggested that delays in accessing workers' compensation and disability insurance may have impact on productivity outcomes among working age patients with chronic pain.15,45
Therefore, future economic evaluation studies should also incorporate presenteeism as a measure to capture productivity loss. An assessment of the presence and time taken to access workers' compensation and disability insurance will also help strengthen the societal perspective of these studies. Future cost-effectiveness studies of pain management treatments should also include a measure of the return-to-work outcome for the working age population while capturing improvements in the quality of life after attending the pain intervention.
Among the included studies, most followed patients for up to one year, with 231,42 following patients for up to 2 years. We recommend additional cost-effectiveness studies with longer-term follow-up measuring productivity for interventions targeted at the working age population or analyse this subset of population in the future. Inclusion of productivity gain/loss in economic evaluations is important from the societal perspective to accurately inform decision makers about the costs (and potential savings) of healthcare interventions.
Given the complex challenges of enabling patients with chronic pain to return to work, it seems that they are likely to require interventions that address these multiple challenges at the individual and workplace levels to support their return to work or maintain labour force participation despite pain. This study aimed to identify economic evaluation studies of chronic pain management interventions quantifying productivity outcomes. This study suggests that there is merit in the inclusion of productivity measures in the base case or additional analyses if future economic evaluations of these interventions are to be undertaken. This will enable decision makers and payers to inform their funding decisions capturing broader societal gains of such interventions.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Acknowledgements
Author contributions: A.R.C. and D.S. conceived and designed the study. A.R.C. and P.L.G. performed the literature search. A.R.C., P.L.G., D.C., and D.S. extracted the data. P.L.G., D.S., and M.N. provided supervision. All authors interpreted the data. A.R.C. drafted the manuscript. All authors contributed to and approved the final manuscript.
The corresponding author received PhD funding from the Pain Foundation (Pain Management Research Institute), University of Sydney, Australia.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Contributor Information
Petra L. Graham, Email: petra.graham@mq.edu.au.
Deborah Schofield, Email: Deborah.schofield@mq.edu.au.
Daniel S.J. Costa, Email: daniel.costa@sydney.edu.au.
Michael Nicholas, Email: michael.nicholas@sydney.edu.au.
References
- [1].Apeldoorn AT, Ostelo RW, van Helvoirt H, Fritz JM, de Vet HC, van Tulder MW. The cost-effectiveness of a treatment-based classification system for low back pain: design of a randomised controlled trial and economic evaluation. BMC Musculoskelet Disord 2010;11:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Apeldoorn AT, Bosmans JE, Ostelo RW, de Vet HC, van Tulder MW. Cost-effectiveness of a classification-based system for sub-acute and chronic low back pain. Eur Spine J 2012;21:1290–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Berger A, Dukes EM, Oster G. Clinical characteristics and economic costs of patients with painful neuropathic disorders. J Pain 2004;5:143–9. [DOI] [PubMed] [Google Scholar]
- [4].Bergström G, Bergström C, Hagberg J, Bodin L, Jensen I. A 7-year follow-up of multidisciplinary rehabilitation among chronic neck and back pain patients. Is sick leave outcome dependent on psychologically derived patient groups? Eur J Pain 2010;14:426–33. [DOI] [PubMed] [Google Scholar]
- [5].Blyth FM, March LM, Brnabic AJ, Jorm LR, Williamson M, Cousins MJ. Chronic pain in Australia: a prevalence study. PAIN 2001;89:127–34. [DOI] [PubMed] [Google Scholar]
- [6].Chou R, Deyo R, Friedly J, Skelly A, Hashimoto R, Weimer M, Fu R, Dana T, Kraegel P, Griffin J, Grusing S, Brodt E. Noninvasive treatments for low back pain. Rockville, MD: Agency for Healthcare Research and Quality (US), 2016. [PubMed] [Google Scholar]
- [7].Chowdhury AR, Graham PL, Schofield D, Cunich M, Nicholas M. Cost-effectiveness of multidisciplinary interventions for chronic low back pain: a narrative review. Clin J Pain 2021;38:197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chowdhury AR, Graham PL, Schofield D, Nicholas M. Productivity outcomes from chronic pain management interventions in the working age population; a systematic review protocol.2021.Available at: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021231028. Accessed February 24, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chuang LH, Soares MO, Tilbrook H, Cox H, Hewitt CE, Aplin J, Semlyen A, Trewhela A, Watt I, Torgerson DJ. A pragmatic multicentered randomized controlled trial of yoga for chronic low back pain: economic evaluation. Spine 2012;37:1593–601. [DOI] [PubMed] [Google Scholar]
- [10].Cox H, Tilbrook H, Aplin J, Chuang LH, Hewitt C, Jayakody S, Semlyen A, Soares MO, Torgerson D, Trewhela A, Watt I, Worthy G. A pragmatic multi-centred randomised controlled trial of yoga for chronic low back pain: trial protocol. Complement Ther Clin Pract 2010;16:76–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dahlhamer J, Lucas J, Zelaya C, Nahin R, Mackey S, DeBar L, Kerns R, Von Korff M, Porter L, Helmick C. Prevalence of chronic pain and high-impact chronic pain among adults—United States, 2016. MMWR Morb Mortal Wkly Rep 2018;67:1001–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Deloitte Access Economics. The cost of pain in Australia. 2019. Available at: https://www.painaustralia.org.au/static/uploads/files/the-cost-of-pain-in-australia-final-report-12mar-wfxbrfyboams.pdf. Accessed August 21, 2019. [Google Scholar]
- [13].Fairbank JC, Pynsent PB. The oswestry disability index. Spine (Phila Pa 1976) 2000;25:2940–53; discussion 2952. [DOI] [PubMed] [Google Scholar]
- [14].Fayaz A, Croft P, Langford RM, Donaldson LJ, Jones GT. Prevalence of chronic pain in the UK: a systematic review and meta-analysis of population studies. BMJ Open 2016;6:e010364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gallagher RM, Myers P. Referral delay in back pain patients on worker's compensation. Psychosomatics 1996;37:270–84. [DOI] [PubMed] [Google Scholar]
- [16].Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain 2012;13:715–24. [DOI] [PubMed] [Google Scholar]
- [17].GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1789–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Goossens ME, de Kinderen RJ, Leeuw M, de Jong JR, Ruijgrok J, Evers SM, Vlaeyen JW. Is exposure in vivo cost-effective for chronic low back pain? A trial-based economic evaluation. BMC Health Serv Res 2015;15:549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Henschke N, Maher CG, Refshauge KM, Herbert RD, Cumming RG, Bleasel J, York J, Das A, McAuley JH. Prognosis in patients with recent onset low back pain in Australian primary care: inception cohort study. BMJ 2008;337:a171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Husereau D, Drummond M, Augustovski F, de Bekker-Grob E, Briggs AH, Carswell C, Caulley L, Chaiyakunapruk N, Greenberg D, Loder E, Mauskopf J, Mullins CD, Petrou S, Pwu RF, Staniszewska S; CHEERS 2022 ISPOR Good Research Practices Task Force. Consolidated health economic evaluation reporting standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. Value Health 2022;25:3–9. [DOI] [PubMed] [Google Scholar]
- [21].Kemani MK, Olsson GL, Lekander M, Hesser H, Andersson E, Wicksell RK. Efficacy and cost-effectiveness of acceptance and commitment therapy and applied relaxation for longstanding pain: a randomized controlled trial. Clin J Pain 2015;31:1004–16. [DOI] [PubMed] [Google Scholar]
- [22].Kigozi J, Jowett S, Lewis M, Barton P, Coast J. Estimating productivity costs using the friction cost approach in practice: a systematic review. Eur J Health Econ 2016;17:31–44. [DOI] [PubMed] [Google Scholar]
- [23].Koopmanschap MA, Rutten FF. A practical guide for calculating indirect costs of disease. Pharmacoeconomics 1996;10:460–6. [DOI] [PubMed] [Google Scholar]
- [24].Lambeek LC, Bosmans JE, Van Royen BJ, Van Tulder MW, Van Mechelen W, Anema JR. Effect of integrated care for sick listed patients with chronic low back pain: economic evaluation alongside a randomised controlled trial. BMJ 2010;341:c6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Landén Ludvigsson M, Peolsson A, Peterson G, Dedering Å, Johansson G, Bernfort L. Cost-effectiveness of neck-specific exercise with or without a behavioral approach versus physical activity prescription in the treatment of chronic whiplash-associated disorders: analyses of a randomized clinical trial. Medicine (Baltimore) 2017;96:e7274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Leeuw M, Goossens M, van Breukelen GJP, de Jong JR, Heuts P, Smeets R, Köke AJA, Vlaeyen JWS. Exposure in vivo versus operant graded activity in chronic low back pain patients: results of a randomized controlled trial. PAIN 2008;138:192–207. [DOI] [PubMed] [Google Scholar]
- [27].Methley AM, Campbell S, Chew-Graham C, McNally R, Cheraghi-Sohi S. PICO, PICOS and SPIDER: a comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv Res 2014;14:579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].National Guideline C. NICE Evidence Reviews Collection. Evidence review for social interventions for chronic pain (chronic primary pain and chronic secondary pain): Chronic pain (primary and secondary) in over 16s: assessment of all chronic pain and management of chronic primary pain: Evidence review D. London: National Institute for Health and Care Excellence (NICE) Copyright © NICE; 2021. [PubMed] [Google Scholar]
- [30].Nicholas MK, Costa DSJ, Blanchard M, Tardif H, Asghari A, Blyth FM. Normative data for common pain measures in chronic pain clinic populations: closing a gap for clinicians and researchers. PAIN 2019;160:1156–65. [DOI] [PubMed] [Google Scholar]
- [31].Niemistö L, Rissanen P, Sarna S, Lahtinen-Suopanki T, Lindgren KA, Hurri H. Cost-effectiveness of combined manipulation, stabilizing exercises, and physician consultation compared to physician consultation alone for chronic low back pain: a prospective randomized trial with 2-year follow-up. Spine (Phila Pa 1976) 2005;30:1109–15. [DOI] [PubMed] [Google Scholar]
- [32].Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med 2001;33:337–43. [DOI] [PubMed] [Google Scholar]
- [33].Ruiz-Negrón N, Menon J, King JB, Ma J, Bellows BK. Cost-effectiveness of treatment options for neuropathic pain: a systematic review. Pharmacoeconomics 2019;37:669–88. [DOI] [PubMed] [Google Scholar]
- [34].Schaefer C, Sadosky A, Mann R, Daniel S, Parsons B, Tuchman M, Anschel A, Stacey BR, Nalamachu S, Nieshoff E. Pain severity and the economic burden of neuropathic pain in the United States: BEAT Neuropathic Pain Observational Study. Clinicoecon Outcomes Res 2014;6:483–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Schofield DJ, Shrestha RN, Percival R, Callander EJ, Kelly SJ, Passey ME. Early retirement and the financial assets of individuals with back problems. Eur Spine J 2011;20:731–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Schweikert B, Jacobi E, Seitz R, Cziske R, Ehlert A, Knab J, Leidl R. Effectiveness and cost-effectiveness of adding a cognitive behavioral treatment to the rehabilitation of chronic low back pain. J Rheumatol 2006;33:2519–26. [PubMed] [Google Scholar]
- [37].Sheahan PJ, Nelson-Wong EJ, Fischer SL. A review of culturally adapted versions of the Oswestry Disability Index: the adaptation process, construct validity, test-retest reliability and internal consistency. Disabil Rehabil 2015;37:2367–74. [DOI] [PubMed] [Google Scholar]
- [38].Sintonen H. The 15D instrument of health-related quality of life: properties and applications. Ann Med 2001;33:328–36. [DOI] [PubMed] [Google Scholar]
- [39].Smeets RJ, Severens JL, Beelen S, Vlaeyen JW, Knottnerus JA. More is not always better: cost-effectiveness analysis of combined, single behavioral and single physical rehabilitation programs for chronic low back pain. Eur J Pain 2009;13:71–81. [DOI] [PubMed] [Google Scholar]
- [40].Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- [41].Tait RC, Pollard CA, Margolis RB, Duckro PN, Krause SJ. The Pain Disability Index: psychometric and validity data. Arch Phys Med Rehabil 1987;68:438–41. [PubMed] [Google Scholar]
- [42].Thomas KJ, MacPherson H, Ratcliffe J, Thorpe L, Brazier J, Campbell M, Fitter M, Roman M, Walters S, Nicholl JP. Longer term clinical and economic benefits of offering acupuncture care to patients with chronic low back pain. Health Technol Assess 2005;9:iii–iv, ix-x, 1–109. [DOI] [PubMed] [Google Scholar]
- [43].Treede RD, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, Cohen M, Evers S, Finnerup NB, First MB, Giamberardino MA, Kaasa S, Korwisi B, Kosek E, Lavand'homme P, Nicholas M, Perrot S, Scholz J, Schug S, Smith BH, Svensson P, Vlaeyen JWS, Wang SJ. Chronic pain as a symptom or a disease: the IASP classification of chronic pain for the international classification of diseases (ICD-11). PAIN 2019;160:19–27. [DOI] [PubMed] [Google Scholar]
- [44].Tsuji T, Matsudaira K, Sato H, Vietri J, Jaffe DH. Association between presenteeism and health-related quality of life among Japanese adults with chronic lower back pain: a retrospective observational study. BMJ Open 2018;8:e021160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Turk DC, Okifuji A. Perception of traumatic onset, compensation status, and physical findings: impact on pain severity, emotional distress, and disability in chronic pain patients. J Behav Med 1996;19:435–53. [DOI] [PubMed] [Google Scholar]
- [46].Van den Hout W. The value of productivity in health policy. Appl Health Econ Health Pol 2015;13:311–3. [DOI] [PubMed] [Google Scholar]
- [47].van der Roer N, van Tulder M, van Mechelen W, de Vet H. Economic evaluation of an intensive group training protocol compared with usual care physiotherapy in patients with chronic low back pain. Spine 2008;33:445–51. [DOI] [PubMed] [Google Scholar]
- [48].Vargas C, Bilbeny N, Balmaceda C, Rodríguez MF, Zitko P, Rojas R, Eberhard ME, Ahumada M, Espinoza MA. Costs and consequences of chronic pain due to musculoskeletal disorders from a health system perspective in Chile. Pain Rep 2018;3:e656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Vermeulen SJ, Anema JR, Schellart AJ, van Mechelen W, van der Beek AJ. Cost-effectiveness of a participatory return-to-work intervention for temporary agency workers and unemployed workers sick-listed due to musculoskeletal disorders: design of a randomised controlled trial. BMC Musculoskelet Disord 2010;11:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30:473–83. [PubMed] [Google Scholar]
- [51].Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996;34:220–33. [DOI] [PubMed] [Google Scholar]
- [52].Werner EL, Storheim K, Løchting I, Wisløff T, Grotle M. Cognitive patient education for low back pain in primary care: a cluster randomized controlled trial and cost-effectiveness analysis. Spine 2016;41:455–62. [DOI] [PubMed] [Google Scholar]
- [53].Willich SN, Reinhold T, Selim D, Jena S, Brinkhaus B, Witt CM. Cost-effectiveness of acupuncture treatment in patients with chronic neck pain. PAIN 2006;125:107–13. [DOI] [PubMed] [Google Scholar]