Figure 1. Participants eligible for analysis, nirmatrelvir/ritonavir treatment completion, and propensity score matched selection of untreated participants from a larger case-ascertained household transmission study of COVID-19 in the United States.
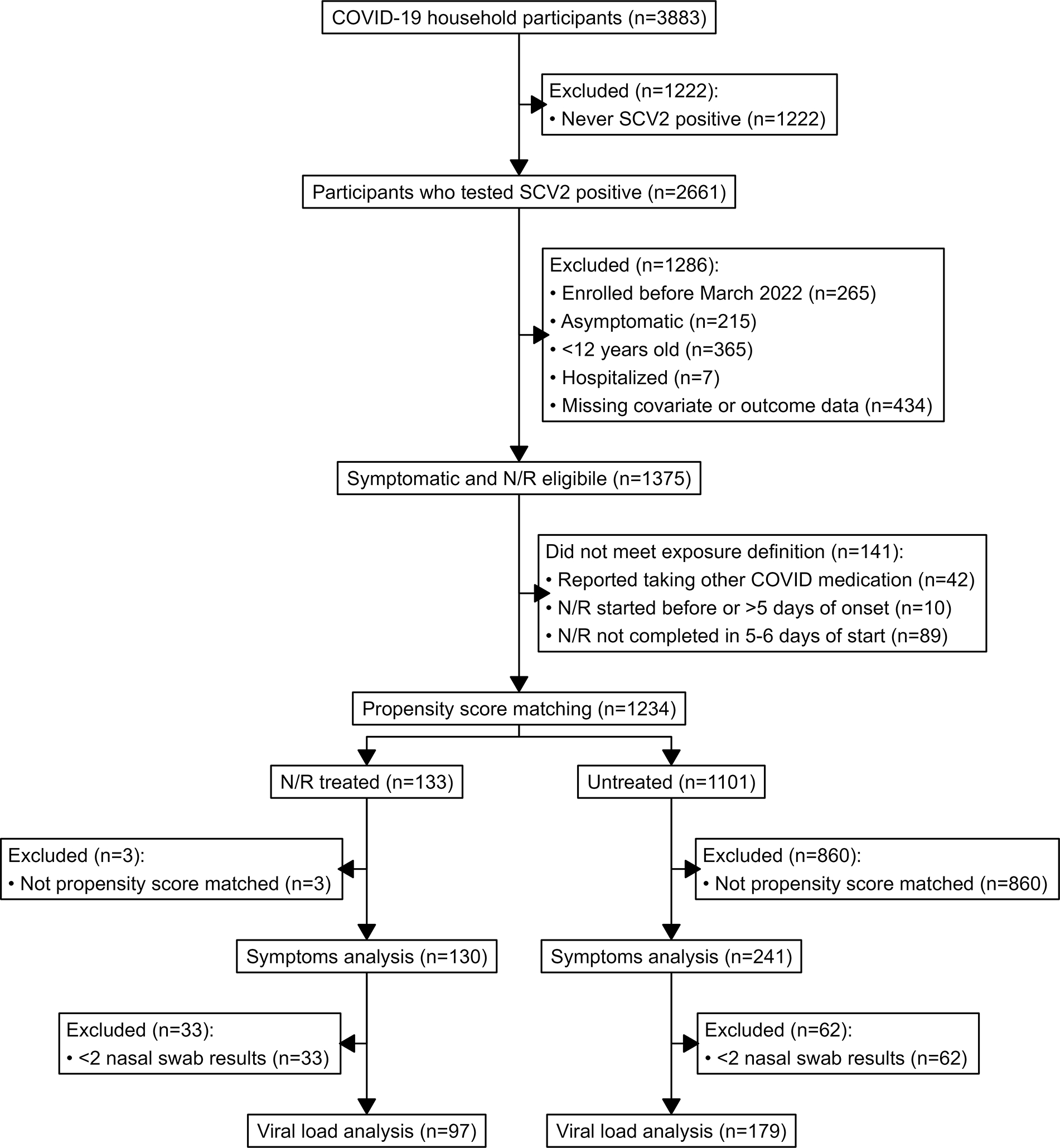
Footnote: Elicited symptoms included fever (including feeling feverish/chills), cough, sore throat, runny nose, nasal congestion, fatigue (including feeling run down), wheezing, trouble breathing (including shortness of breath), chest tightness (including chest pain), loss of smell or taste, headache, abdominal pain, diarrhea, vomiting, and body aches (including muscle aches). Elicited COVID-19 medications included molnupiravir, remdesivir, and nirmatrelvir/ritonavir; other medications were reported in a free-response section of the diary. PS matching was performed using logistic regression with the outcome of N/R treatment completion (N/R treated) versus no COVID-19 treatment (untreated) and covariates included age at enrollment, sex, race/ethnicity, Social Vulnerability Index, prior COVID-19, recruitment method, participant type (index vs. contact), accessed medical care after enrollment, received ≥3 verified COVID-19 vaccine doses, received a verified COVID-19 vaccine dose ≤6 months of index onset, SARS-CoV-2 variant circulating at the time of index onset, number of comorbidities, and whether the participant reported each of asthma or other lung disease, heart disease, diabetes, cancer, liver or kidney disease, immunocompromising condition or taking immunosuppressing medication, or any other chronic health condition. SCV2=SARS-CoV-2; N/R=nirmatrelvir/ritonavir
