Abstract
Pseudomyxoma peritonei (PMP) is a relatively uncommon condition primarily associated with neoplasms of the appendiceal epithelium. It is characterized by non-specific clinical manifestations, leading to a high rate of misdiagnosis. This report describes the case of a 62-year-old male patient with recurrent and metastatic PMP. The patient first experienced unexplained epigastric pain and paroxysmal abdominal pain accompanied by distension over 8 years ago. He underwent surgical interventions for the condition in other hospitals in 2015 and 2018, respectively.
Keywords: Pseudomyxoma peritonei, Recurrence, Metastasis, CT
Introduction
Pseudomyxoma peritonei (PMP) is an uncommon medical condition, with an incidence rate of approximately 3-4 cases per million individuals annually [1]. This condition is characterized by the dissemination of tumorous cells within the peritoneal cavity, leading to the production of mucinous ascites [2]. The majority of PMP cases originate from ruptured appendiceal neoplasms, though a minority stem from mucinous tumors of the ovary, fallopian tube, urachus, colorectum, gallbladder, stomach, pancreas, lung, and breast [3], [4], [5], [6], [7], [8]. Metastasis of PMP beyond the peritoneal cavity via hematogenous or lymphatic routes is rare. The primary pathways of metastasis include (1) implantation of tumorous cells into the greater and lesser omentum, the junction of the small intestine and its mesentery, and the right subdiaphragmatic region due to peritoneal fluid reflux; (2) extensive involvement of the peritoneal cavity influenced by gravitational forces; and (3) implantation and spread of tumorous cells following surgical interventions and rupture of lesions. The omentum and right subdiaphragmatic area are the most frequently affected metastatic sites.
Case description
A 62-year-old male with no prior history of tumors presented with unexplained epigastric pain and intermittent episodes of abdominal distension lasting more than 8 years. Initially admitted to our hospital in 2015, he underwent no specific treatment during that visit. Subsequent diagnostic efforts included a computed tomography (CT) scan of the abdomen and pelvis with contrast, revealing multiple low-density masses encircling the liver and spleen, as well as within the greater and lesser omentum (Fig. 1). The patient underwent surgical excision of the identified masses in other hospitals in 2015 and again in 2018. In 2023, the patient returned to our hospital, where a repeat CT scan of the abdomen and pelvis with contrast disclosed diffuse low-density masses surrounding the liver, spleen, greater and lesser omentum, mesentery, and pelvic cavity, with scattered calcifications and slight enhancement upon contrast administration. The imaging revealed deformation of the liver and spleen due to pressure, evidenced by hepatic and splenic scalloping. Additionally, multiple low-density cystic lesions with partial marginal calcification were observed in both liver and spleen. Compression of the gastrointestinal tract, pancreas, and bladder was notably apparent (Fig. 2). Surgical "resection of peritoneal and retroperitoneal lesions" was subsequently performed at our hospital. The surgical findings suggested an appendiceal myxoma origin. Histopathological examination using light microscopy identified extensive mucin adhesion within the pathological tissue, with visible mucinous cells, some exhibiting fine papillary structures and basally located rod-shaped nuclei (Fig. 3). Immunohistochemical staining yielded the following results: Ki-67 (+, about5%), MLH1 (+), MSH2 (+), MSH6 (+), P53 (+), PMS2 (+). Based on these findings, the patient was diagnosed with low-grade pseudomyxoma peritonei.
Fig. 1.
In 2015, the patient underwent a CT scan of the abdomen and pelvis with contrast for the first time. The findings showed multiple low-density masses adjacent to the liver and spleen and within the greater and lesser omentum. The masses demonstrated CT attenuation values between 18 and 26 HU, with no clear calcification. Post-contrast images reveal no significant enhancement. Additionally, effusions were observed in both abdominal and pelvic cavities.
Fig. 2.
Contrast-enhanced CT scan of the abdomen and pelvis in 2023 illustrating diffuse low-density masses encircling the liver and spleen, within the greater and lesser omentum, mesentery, and pelvic cavity. The lesions exhibited CT attenuation values between 16 to 28 HU, with scattered areas of calcification and mild enhancement following contrast administration. Compression-induced deformities of the liver and spleen were evident, manifesting as hepatic and splenic scalloping. Additionally, multiple low-density cystic lesions with partial marginal calcification were noted in both the liver and spleen. The gastrointestinal tract, pancreas, and bladder appeared significantly compressed. No substantial retroperitoneal lymph node enlargement was observed, nor were there definitive signs of bone metastasis on the scan.
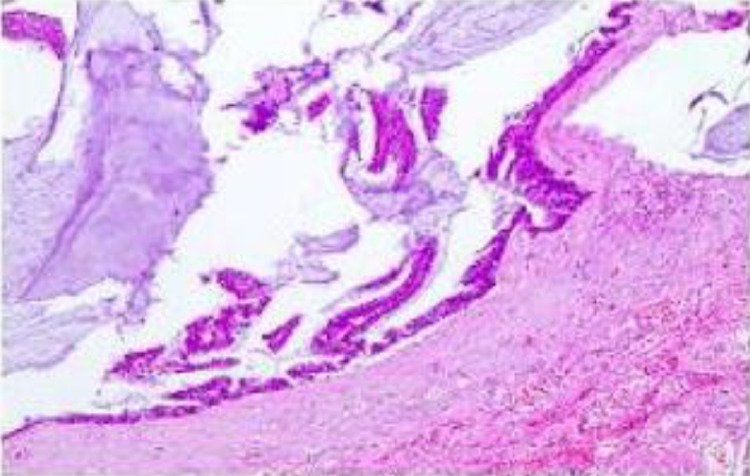
Fig. 3.
Histological findings from a biopsy performed in 2023 demonstrating significant mucinous adhesion within the pathological specimen. Visible within the mucus were mucinous cells, some of which form fine papillary structures with basally located rod-shaped nuclei. These features were indicative of low-grade pseudomyxoma peritonei.
Discussion
Pseudomyxoma peritonei (PMP) is a highly rare condition characterized by mucinous ascites and peritoneal implants, a process known as the "redistribution phenomenon" [9]. The disease progresses slowly and exhibits non-specific clinical symptoms, which, combined with the general lack of awareness among clinicians and radiologists, leads to a high rate of misdiagnosis. Typical clinical symptoms include abdominal pain, distension, and the presence of an abdominal mass. As the disease advances, patients may experience incomplete intestinal obstruction, cachexia, significant ascites, and reduced urine output. PMP varies widely in its clinical behavior, ranging from indolent benign lesions to aggressively invasive disease, and is thus classified as a borderline malignant tumor [2]. In 1995, Ronnett et al. first categorized PMP into 3 distinct types: disseminated peritoneal adenomucinosis (DPAM), peritoneal mucinous carcinomatosis (PMCA), and peritoneal mucinous carcinomatosis with intermediate features (PMCA-I) [10]. More recently, in 2016, the Peritoneal Surface Oncology Group International (PSOGI) updated the classification into 4 types: acellular mucin, low-grade mucinous carcinoma peritonei (equivalent to DPAM), peritoneal mucinous carcinomatosis (PMCA), and high-grade mucinous carcinoma peritonei with signet ring cells [11]. Typically, DPAM originates from benign lesions, such as appendiceal mucinous adenomas, appendiceal mucinous cysts, ovarian mucinous cystadenomas, and ovarian mucinous cystomas. Conversely, PMCA is usually derived from malignant lesions like appendiceal mucinous cystadenocarcinomas and ovarian mucinous cystadenocarcinomas. Following rupture, mucinous lesions from the appendix or ovary release mucin containing tumor cells, which then spread extensively within the peritoneal cavity, leading to widespread metastasis.
CT stands as the most prevalent imaging modality for the diagnosis, classification, and localization of PMP. CT imaging proficiently delineates the distribution of PMP, closely paralleling the findings of laparotomy exploration, and thus holds significant value for clinicians in formulating treatment strategies and monitoring for recurrence.
Radiologists must recognize the distinctive characteristics of PMP on CT scans. The condition typically presents as low-density areas within the peritoneal cavity, with CT values akin to or slightly above that of water, likely due to the presence of gelatinous masses. Scattered calcifications and septations may be visible within these low-density regions. The lesions exhibit no enhancement or demonstrate separation with mild enhancement upon contrast-enhanced scanning. As the disease advances, additional signs such as hepatic and splenic scalloping, separation of intestinal loops, and infiltration of the peritoneum and omentum become evident on CT [12], [13].
Similar to CT, magnetic resonance imaging (MRI) reveals multiple cystic lesions within the peritoneal cavity and scalloping changes on the margins of the liver, spleen, and other organs. Although MRI has inferior spatial resolution compared to CT, its superior soft tissue contrast allows for an enhanced assessment of tumor extent. Consequently, combining CT and MRI provides a more comprehensive evaluation of the lesion's involvement [14].
Differential diagnosis of diffuse PMP includes malignant peritoneal mesothelioma, peritoneal metastasis, tuberculous peritonitis, and peritoneal effusion, while localized PMP should be differentiated from pancreatic pseudocysts and lymphatic cysts.
Surgery remains the primary treatment for PMP. Historically, traditional surgery, focusing on mere tumor debulking, offered temporary symptom relief but was associated with high rates of postoperative recurrence, necessitating multiple interventions and resulting in poor prognosis. The introduction of combined complete cytoreductive surgery (CCRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) in the 1990s significantly enhanced treatment outcomes, elevating the 10-year survival rates from 30% to between 63% and 74%. This combination has now become the most effective approach for managing PMP [15], [16].
Other therapeutic methods for pseudomyxoma peritonei include radiotherapy, immunotherapy, and biotherapy, although their utilization in clinical practice is limited. The existing literature on radiotherapy for pseudomyxoma peritonei is scarce. Some studies have indicated that radiotherapy is employed in cases of advanced and recurrent disease, it has been observed to decrease the size of lesions, minimizing the need for surgical intervention, and can be beneficial for managing symptomatic intestinal obstruction [17], [18], [19]. McGrath et al. documented positive outcomes with low-dose palliative radiotherapy in pseudomyxoma peritonei patients [20]. However, a retrospective analysis conducted by Gough et al. demonstrated that the combination of surgery with intracavity or external radiotherapy did not yield improvements in overall survival rates [21]. Given the limited clinical experience with radiotherapy in pseudomyxoma peritonei, further research is necessary to ascertain its efficacy.
Conclusions
PMP is a unique clinicopathological entity characterized by the accumulation of mucinous ascites within the peritoneal cavity, primarily arising from appendiceal tumors. Its clinical behavior mirrors that of a malignant process due to its propensity for recurrence and adhesion, alongside its chronic debilitating nature. Currently, the optimal therapeutic strategy involves a combination of CCRS and HIPEC. Imaging modalities, such as MR and CT of the abdomen and pelvis, play a crucial role in the early detection of PMP. These imaging techniques provide comprehensive visualization of the extent of lesion involvement, including the affected abdominal organs and potential vascular invasion, thereby facilitating the formulation of effective treatment plans.
Ethics approval
The study involving human subject was reviewed and approved by the Medical Research Ethics Committee of Hainan General Hospital in accordance with the Helsinki Declaration.
Patient consent
In this retrospective study, this patient's written informed consent was obtained.
Footnotes
Competing Interests: The authors declare that there is no conflict of interest.
References
- 1.Patrick-Brown TDJH, Carr NJ, Swanson DM, Larsen S, Mohamed F, Flatmark K. Estimating the prevalence of pseudomyxoma peritonei in Europe using a novel statistical method. Ann Surg Oncol. 2021;28(1):252–257. doi: 10.1245/s10434-020-08655-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moran BJ, Cecil TD. The etiology, clinical presentation, and management of pseudomyxoma peritonei. Surg Oncol Clin N Am. 2003;12(3):585–603. doi: 10.1016/s1055-3207(03)00026-7. [DOI] [PubMed] [Google Scholar]
- 3.Smeenk RM, van Velthuysen ML, Verwaal VJ, Zoetmulder FA. Appendiceal neoplasms and pseudomyxoma peritonei: a population based study. Eur J Surg Oncol. 2008;34:196–201. doi: 10.1016/j.ejso.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 4.McCarthy JH, Aga R. A fallopian tube lesion of borderline malignancy associated with pseudo-myxoma peritonei. Histopathology. 1988;13(2):223–225. doi: 10.1111/j.1365-2559.1988.tb02028.x. [DOI] [PubMed] [Google Scholar]
- 5.Giang TH, Ngoc TT, Hassell LA. Carcinoma involving the gallbladder: a retrospective review of 23 cases - pitfalls in diagnosis of gallbladder carcinoma. Diagn Pathol. 2012;7:10. doi: 10.1186/1746-1596-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ikejiri K, Anai H, Kitamura K, Yakabe S, Saku M, Yoshida K. Pseudomyxoma peritonei concomitant with early gastric cancer: report of a case. Surg Today. 1996;26:923–925. doi: 10.1007/BF00311797. [DOI] [PubMed] [Google Scholar]
- 7.Goldin M, Li J, Amirrezvani A, Riker D. Pulmonary giant cell carcinoma associated with pseudomyxoma peritonei. J Bronchology Interv Pulmonol. 2012;19(1):50–53. doi: 10.1097/LBR.0b013e318244294b. [DOI] [PubMed] [Google Scholar]
- 8.Hawes D, Robinson R, Wira R. Pseudomyxoma peritonei from metastatic colloid carcinoma of the breast. Gastrointest Radiol. 1991;16(1):80–82. doi: 10.1007/BF01887311. [DOI] [PubMed] [Google Scholar]
- 9.Sugarbaker PH. Pseudomyxoma peritonei. A cancer whose biology is characterized by a redistribution phenomenon. Ann Surg. 1994;219(2):109–111. doi: 10.1097/00000658-199402000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ronnett BM, Zahn CM, Kurman RJ, Kass ME, Sugarbaker PH, Shmookler BM. Disseminated peritoneal adenomucinosis and peritoneal mucinous carcinomatosis. A clinicopathologic analysis of 109 cases with emphasis on distinguishing pathologic features, site of origin, prognosis, and relationship to "pseudomyxoma peritonei". Am J Surg Pathol. 1995;19(12):1390–1408. doi: 10.1097/00000478-199512000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Carr NJ, Cecil TD, Mohamed F, Sobin LH, Sugarbaker PH, González-Moreno S, et al. A consensus for classification and pathologic reporting of pseudomyxoma peritonei and associated appendiceal neoplasia: the results of the Peritoneal Surface Oncology Group International (PSOGI) modified Delphi process. Am J Surg Pathol. 2016;40(1):14–26. doi: 10.1097/PAS.0000000000000535. [DOI] [PubMed] [Google Scholar]
- 12.Parikh VP, Jain C, Desai MB. CT of pseudomyxoma peritonei. AJR Am J Roentgenol. 1987;149(5):1077–1078. doi: 10.2214/ajr.149.5.1077. [DOI] [PubMed] [Google Scholar]
- 13.Hotta M, Minamimoto R, Gohda Y, Tajima T, Kiyomatsu T, Yano H. Pseudomyxoma peritonei: visceral scalloping on CT is a predictor of recurrence after complete cytoreductive surgery. Eur Radiol. 2020;30(8):4193–4200. doi: 10.1007/s00330-020-06756-2. [DOI] [PubMed] [Google Scholar]
- 14.Menassel B, Duclos A, Passot G, Dohan A, Payet C, Isaac S, et al. Preoperative CT and MRI prediction of non-resectability in patients treated for pseudomyxoma peritonei from mucinous appendiceal neoplasms. Eur J Surg Oncol. 2016;42(4):558–566. doi: 10.1016/j.ejso.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Chua TC, Moran BJ, Sugarbaker PH, Levine EA, Glehen O, Gilly FN, et al. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol. 2012;30(20):2449–2456. doi: 10.1200/JCO.2011.39.7166. [DOI] [PubMed] [Google Scholar]
- 16.Ansari N, Chandrakumaran K, Dayal S, Mohamed F, Cecil TD, Moran BJ. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in 1000 patients with perforated appendiceal epithelial tumors. Eur J Surg Oncol. 2016;42(7):1035–1041. doi: 10.1016/j.ejso.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez RN, Daly JM. Pseudomyxoma peritonei. Arch Surg. 1980;115:409–414. doi: 10.1001/archsurg.1980.01380040037006. [DOI] [PubMed] [Google Scholar]
- 18.El Sayed S. Pseudomyxoma peritonei treated by radiotherapy. Clin Oncol (R Coll Radiol) 1990;2:120–122. doi: 10.1016/s0936-6555(05)80801-5. [DOI] [PubMed] [Google Scholar]
- 19.Berkovic P, van de Voorde L, De Meerleer G, Delrue L, Speleers B, Van Belle S, et al. Whole abdominopelvic radiotherapy in the palliative treatment of pseudomyxoma peritonei. Strahlenther Onkol. 2014;190:223–228. doi: 10.1007/s00066-013-0470-7. [DOI] [PubMed] [Google Scholar]
- 20.McGrath C, Linden K, Hube P, Adamiak A, Dennis K. Palliative radiation therapy for symptom control in an advanced case of pseudomyxoma peritonei. Cureus. 2017;9(6):e1407. doi: 10.7759/cureus.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gough DB, Donohue JH, Schutt AJ, Gonchoroff N, Goellner JR, Wilson TO, et al. Pseudomyxoma peritonei. Long-term patient survival with an aggressive regional approach. Ann Surg. 1994;219(2):112–119. doi: 10.1097/00000658-199402000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]