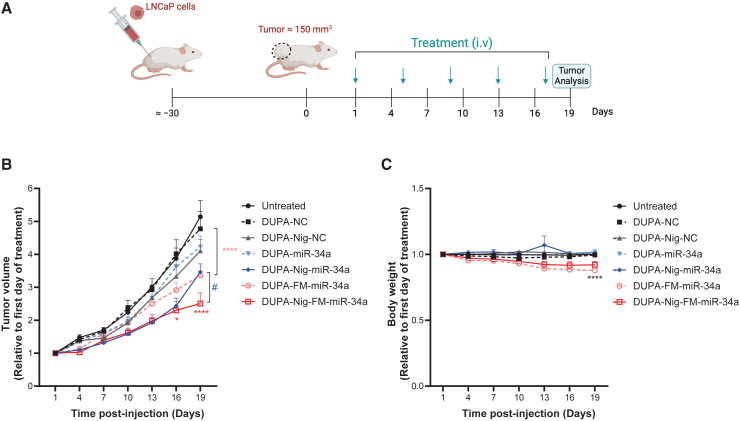
Figure 6.
DUPA-Nig-FM-miR-34a treatment decrease the tumor burden of LNCaP xenografts
(A) Schematic of the treatment schedule. (B) Tumor volumes of LNCaP tumor following intravenous administration of DUPA-miR-34a, DUPA-nigericin-miR-34a, DUPA-FM-miR-34a, DUPA-nigericin-FM-miR-34a, or respective controls (n = 6 mice per group, 4 nmol, intravenous injection, once every 4 days). Error bars are means ± SEM, ∗p < 0.05, ∗∗∗∗p < 0.0001, #p < 0.05; two-way ANOVA significance determined against respective NC (DUPA-Nig-FM-miR-34a relative to DUPA-Nig-NC; DUPA-miR-34a relative to DUPA-NC) is indicated by asterisks colored relative to the group being compared; significance determined between DUPA-Nig-miR-34a and DUPA-Nig-FM-miR-34a is indicated by #. (C) Body weight measurement throughout the treatment period. Error bars are means ± SEM. ∗∗∗∗p < 0.0001; two-way ANOVA. On day 19, significance was determined against the respective NC treatment group.