Abstract
Background:
Weakness can be operationalized with several thresholds, which in turn, could impact associations with cognitive impairment when considering obesity status.
Objective:
We examined the associations of absolute, normalized, and collective weakness thresholds on future cognitive impairment by obesity status in older adults.
Methods:
We performed a secondary data analysis on the 2006–2018 waves of the Health and Retirement Study. A spring-type dynamometer collected handgrip strength (HGS). Males were categorized weak if their HGS was <35.5-kg (absolute), <0.45-kg/kg (body mass normalized), or <1.05-kg/kg/m2 (body mass index (BMI) normalized), while females were defined as weak if their HGS was <20.0-kg, <0.337-kg/kg, or <0.79-kg/kg/m2. The modified Telephone Interview of Cognitive Status examined cognitive function. Persons scoring ≤10 had a cognitive impairment. Obesity was categorized as BMI ≥30 kg/m2.
Results:
We included 7,532 and 3,584 persons aged ≥65-years living without and with obesity, respectively. Those without obesity but beneath the absolute weakness threshold had 1.54 (95% confidence interval (CI): 1.24–1.91) greater odds for future cognitive impairment. Persons with obesity and beneath each threshold also had greater odds for future cognitive impairment: 1.89 (95% CI: 1.28–2.78) for absolute, 2.17 (95% CI: 1.02–4.62) for body mass normalized, and 1.75 (95% CI: 1.10–2.80) for BMI normalized. Older Americans without obesity but underneath all the weakness thresholds had 1.32 (95% CI: 1.00–1.74) greater odds for impairment in cognitive function, while persons with obesity had 2.76 (95% CI: 1.29–5.93) greater odds.
Conclusions:
There should be consideration for how body size and different weakness thresholds may influence future cognitive outcomes.
Keywords: Aging, Alzheimer’s disease, body composition, body mass index, cognition, dementia, functional status, geriatrics, muscle strength, muscle strength dynamometer
INTRODUCTION
Approximately 67% of Americans will experience some level of cognitive impairment [1], and Alzheimer’s disease (AD) is present in an estimated 6.7 million older Americans [2]. The number of older Americans living with a cognitive impairment is projected to increase, and AD/Alzheimer’s disease related dementias (AD/ADRD) is a leading cause of death [2]. Although many risk factors exist for AD/ADRD including an elevated risk at age ≥65-years that potentiates at ≥75-years [3], obesity and muscle-related conditions are associated with cognitive impairment [4, 5]. Therefore, body mass index (BMI) and handgrip strength (HGS) have been discussed as viable screening factors to help identify risk for future cognitive impairment, and for targeted interventions to mitigate such risk.
Although several thresholds exist for determining weakness from HGS, the Sarcopenia Definitions and Outcomes Consortium (SDOC) created absolute and normalized thresholds for weakness [6, 7]. Indeed, normalized cut-points help to provide a stand-alone adjustment of body size for strength capacity, thereby providing precision in measurement, but the quotients derived from these adjustments should be closely observed. For example, concerns for how to interpret longitudinal changes in normalized HGS measures may exist because it is unclear if body size or strength capacity (or both) are driving changes [8]. Therefore, understanding how absolute and body size normalized weakness thresholds linked to cognitive functioning by obesity status present guidance for determining how body size might influence such associations.
While the SDOC has generated weakness classifications from absolute and normalized HGS, opportunities to utilize these classifications together may also exist to better operationalize weakness for prognosis of deteriorating health. For example, previous studies have shown that the odds of future falls and cognitive impairment elevate when persons are beneath absolute and body size normalized weakness thresholds [9, 10], and the proportions of individuals below these weakness thresholds are not equivalent [11]. Given that the SDOC cut-points are emerging as a rigorous method for defining low strength capacity, examining the role of obesity with respect to these thresholds may provide additional guidance into how weakness is associated with cognitive impairment. Following framework from a previous investigation on this topic [9], the purpose of this study was to determine the associations of individual absolute and body size normalized weakness thresholds on cognitive impairment by obesity status in older Americans. We also utilized these individual weakness thresholds collectively in older Americans to determine future cognitive impairment by obesity status.
MATERIALS AND METHODS
Dataset
The 2006–2018 waves of the RAND Health and Retirement Study were analyzed for this investigation. We extracted data from 11,242 participants that were at least 65 years of age with information for ≥1 wave of HGS, and at least another wave of cognitive function assessment. The HRS observes age-related health factors in Americans using a longitudinal-panel design [12]. Participants from the HRS often complete measures every other year until death, and refreshment samples help the HRS maintain a national sample [13]. Interview response rates for the HRS have repeatedly been >80% [14]. Additional information regarding the HRS is published elsewhere [15].
The HRS started performing detailed face-to-face interviews with physical function measures such as HGS beginning in the 2006 wave [13]. These thorough interviews occurred on a random sub-sample of half the HRS participants, with these interviews occurring every other wave, while the other half sample of participants only completed the routine interviews to support study execution. Written informed consent was given by HRS participants and study procedures were approved by the University of Michigan Health Sciences/Behavioral Sciences Institutional Review Board.
Measurements
Cognitive assessment
A modified form of the validated Telephone Interview of Cognitive Status was utilized to assess cognitive function [16]. The modified Telephone Interview of Cognitive Status, which is a cognitive screener modeled from the Mini-Mental State Examination, has the ability to abate ceiling and floor effects [17, 18]. We utilized a composite scale wherein scores ranged from 0–35 points, with greater scores suggesting higher cognitive functioning. Evaluations from the modified Telephone Interview of Cognitive Status included immediate and delayed word recall, serial sevens subtraction beginning at the number 100, counting backward as quickly as possible starting from the number 20, object and date naming, and correctly reporting the president and vice president of the United States. Participants scoring ≤10 were categorized as having a cognitive impairment [19].
Handgrip strength
Participants eligible for HGS testing did not report having surgery, swelling, harsh pain, or an injury to either hand in the 6-months prior to the interview, and if such symptoms were present in a single hand, then only the other hand was used [20]. The HRS uses a Smedley spring-type hand dynamometer (Scandidact; Odder, Denmark) for measuring HGS [20]. Protocols for measuring HGS in the HRS first begin with fitting the dynamometer to the participant’s hand, and then allowing for a practice trial for familiarity on the reported dominant hand while standing with their arm positioned at a 90° angle on the side [20]. All participants are advised to squeeze the dynamometer as hard as possible for a couple seconds, and then release [20]. The handgrip dynamometer is then placed in the non-dominant hand, and HGS values are recorded twice on each hand, alternating hands [20]. If HGS testing was only conducted on a single hand, then a 30-s rest period was elapsed between measurements [20]. Protocol alternatives were allowed for participants unable to stand or grasp the dynamometer, such that persons could be seated and place their arm on a supporting object [20].
The highest HGS documented despite hand of origin was included in the analyses. Males and females were classified as having weakness if their absolute HGS was below 35.5-kg and 20.0-kg, respectively [6, 11]. Adjustments for HGS to body mass helped to further classify weakness, such that males < 0.45 kg/kg and females < 0.337 kg/kg were weak [6, 11]. Likewise, normalization for HGS to BMI categorized weakness for males at <1.05 kg/kg/m2 and females at <0.79 kg/kg/m2 [6, 11]. To unite these absolute and body size normalized weakness thresholds (i.e., collective weakness), we also categorized participants as being underneath 1, 2, or all 3 thresholds. HGS measurements are frequently used to operationalize weakness [21, 22].
Covariates
Participants self-reported their age, sex, race, educational attainment, height, and body mass. Participants with a BMI≥30 kg/m2 were classified as obese [23]. Each participant likewise indicated if they were current or previous cigarette smokers. Persons with multimorbidity reported an affirmative healthcare provider diagnosis for 2 or more of the following conditions: high blood pressure, diabetes, cancer (excluding minor skin cancer), chronic lung disease (e.g., emphysema), stroke, heart condition (e.g., coronary heart disease, myocardial infarction, angina, congestive heart failure), emotional or psychiatric problems, and arthritis or rheumatism. Perceived health was self-rated by participants from 5 categories: excellent, very good, good, fair, and poor.
Depressive symptoms were measured with the 8-item Center for the Epidemiologic Studies Depression scale [24]. Respondents revealed if they felt positive or negative emotions the week before completing scale. Scores ranged from 0–8, with greater scores indicating depressive symptomology was higher. We classified participants as engaging in moderate-to-vigorous physical activity (MVPA) if they reported such physical activity participation for at least a single occasion during the week [25]. Multiple items were used to ascertain social participation: 1) spent time doing volunteer work in the past 12 months for religions, educational, health-related, or other charitable organizations, 2) weekly communications with parents, in-laws, or partners, and 3) current employment status. Scores ranged from 0–3 with greater scores suggesting more engagement in social activities [26]. Participants reported on their ability to use a toilet, dress (including putting on shoes and socks), eat (such as cutting-up food), get in-and-out of bed, bathe or shower, and walk across a room. Those indicating difficulty or incapability in completing an activity of daily living (ADL) were classified as having an ADL limitation. Individuals that had missingness for covariates were not included (n = 126).
Statistical approach
SAS 9.4 software (SAS Institute; Cary, NC) was used to execute the analyses. Details about the data structuring and measures collected during the longitudinal study period are available elsewhere [9]. Baseline descriptive characteristics of the participants were shown as mean±standard deviation and frequency (proportion) for categorized variables by obesity status. Means and 95% confidence intervals (CI) were similarly generated for the baseline demographic participant characteristics by obesity presence to make comparisons between groups.
A generalized estimating equation (GEE) quantified the association between persons categorized as weak with the absolute threshold (reference: not below absolute weakness threshold) and future cognitive impairment. Another GEE analyzed the association between participants classified as weak with the BMI normalized weakness thresholds (reference: not below the BMI normalized weakness threshold) and future cognitive impairment. Moreover, a GEE determined the association between older Americans classified as weak with the body mass normalized weakness thresholds (reference: not below the body mass normalized weakness threshold) and future cognitive impairment. To evaluate the role of the collective weakness classifications, a GEE examined the association between participants underneath 1, 2, or all 3 weakness thresholds (reference: below 0 weakness thresholds) on future cognitive impairment.
The GEEs were adjusted for cognitive impairment at current wave, follow-up time, sex, race, multimorbidity, age, cigarette smoking status, social activities, perceived health, depressive symptoms, MVPA, ADLs, and educational attainment. Covariates were examined at the current wave. Each GEE controlled for repetitive measures and the outcome for the next wave participated was used. An α-level of 0.05 was utilized.
RESULTS
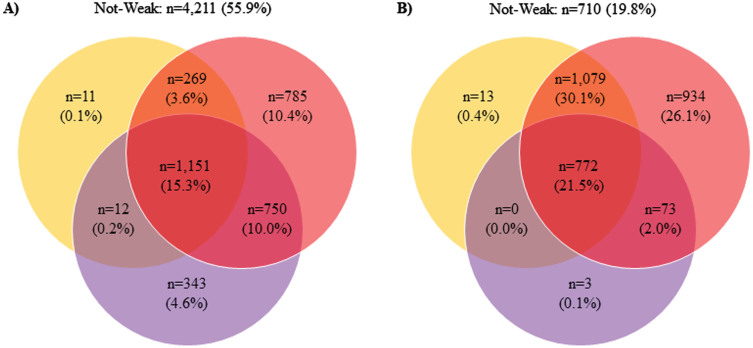
Table 1 shows the baseline participant descriptive characteristics by obesity presence. Of the included 11,116 participants at baseline, 3,584 (32.2%) were living with obesity. Persons with obesity were also aged 70.7±5.4 years, while persons without obesity were aged 73.1±6.8 years. Supplementary Table 1 reveals the means and 95% CIs for the baseline participant descriptive characteristics by obesity status. Figure 1 depicts the number of participants beneath the weakness thresholds by obesity status. There were differences in the number of participants classified as weak relative to the threshold utilized.
Table 1.
Baseline descriptive characteristics of the participants by obesity status
| No Obesity (n = 7,532) | Obesity (n = 3,584) | |
| Age (y) | 73.1±6.8 | 70.7±5.4 |
| Handgrip strength (kg) | 29.6±10.3 | 30.8±10.4 |
| Body mass index (kg/m2) | 25.1±2.9 | 34.6±4.5 |
| Social engagement | 1.2±0.8 | 1.2±0.6 |
| Female (n (%)) | 4,324 (57.4) | 2,135 (59.5) |
| White (n (%)) | 6,327 (84.0) | 2,726 (76.0) |
| Multimorbidity (n (%)) | 4,747 (63.0) | 2,856 (79.7) |
| Cigarette smoking status (n (%)) | ||
| Current smoker | 880 (11.7) | 248 (6.9) |
| Previous smoker | 3,376 (44.8) | 1,758 (49.0) |
| Never smoked | 3,276 (43.5) | 1,578 (44.0) |
| Self-rated health (n (%)) | ||
| Excellent | 842 (11.2) | 1.73 (4.8) |
| Very good | 2,556 (33.9) | 932 (26.0) |
| Good | 2,434 (32.3) | 1,300 (36.3) |
| Fair | 1,350 (17.9) | 898 (25.1) |
| Poor | 350 (4.7) | 281 (7.8) |
| Depressive symptoms | 1.1±1.7 | 1.4±1.9 |
| Moderate-to-vigorous physical activity participation (n (%)) | 4,713 (62.5) | 1,683 (46.9) |
| Activities of daily living limitation (n (%)) | 938 (12.4) | 796 (22.2) |
| High school graduate or above (n (%)) | 6,056 (80.4) | 2,759 (76.9) |
| Follow-up years | 2.1±0.4 | 2.1±0.4 |
| Cognitive impairment (n (%)) | 111 (1.4) | 54 (1.5) |
| Cognitive impairment at next wave (n (%)) | 268 (3.5) | 84 (2.3) |
Fig. 1.
Proportions of Participants Beneath the Weakness Thresholds. A) without obesity; B) with obesity; orange = body mass index normalized weakness; purple = absolute weakness; red = body mass normalized weakness.
Table 2 displays the results for the associations of the individual weakness thresholds on future cognitive impairment by obesity status. For persons living without obesity, only those beneath the absolute weakness threshold had 1.54 (95% CI: 1.24–1.91) greater odds for future cognitive impairment, but there were no observed significant associations for those under the BMI normalized weakness (odds ratio (OR): 1.06; 95% CI: 0.83–1.34) and body mass normalized thresholds (OR: 1.07; 95% CI: 0.86–1.32) regarding those living without obesity. However, participants with obesity that were classified as weak with respect to each threshold had greater odds for future cognitive impairment: 1.89 (95% CI: 1.28–2.78) for absolute, 1.75 (95% CI: 1.10–2.80) for BMI normalized, and 2.17 (95% CI: 1.02–4.62) for body mass normalized weakness.
Table 2.
Results for the Associations of the Individual Weakness Thresholds on Future Cognitive Impairment by Obesity Status
| No Obesity | Obesity | |||
| OR | 95% CI | OR | 95% CI | |
| Absolute Weakness† | 1.54 | 1.24, 1.91 | 1.89 | 1.28, 2.78 |
| Body Mass Index Normalized Weakness‡ | 1.06 | 0.83, 1.34 | 1.75 | 1.10, 2.80 |
| Body Mass Normalized Weakness¥ | 1.07 | 0.86, 1.32 | 2.17 | 1.02, 4.62 |
†Reference: no absolute weakness; ‡Reference: no body mass index normalized weakness; ¥Reference: no body mass normalized weakness. CI, confidence interval; OR, odds ratio. The models controlled for cognitive impairment at current wave, follow-up years, sex, race, multimorbidity, age, smoking status, social engagement, self-rated health, depressive symptoms, moderate-to-vigorous physical activity participation, basic self-care limitation, and educational attainment.
The results for the associations of persons beneath 1, 2, or all 3 weakness thresholds future cognitive impairment by obesity status are presented in Table 3. Only participants living without obesity but beneath each of the 3-weakness thresholds had 1.32 (95% CI: 1.00–1.74) greater odds for future cognitive impairment. However, we observed no significant associations for those below 1 (OR: 1.17; 95% CI: 0.88–1.55) or 2 (OR: 1.20; 95% CI: 0.89–1.61) thresholds. A similar trend was found for persons with obesity, such that only participants beneath all 3-weakness thresholds had 2.76 (CI: 1.29–5.93) greater odds for future cognitive impairment, but again, we found no significant associations those underneath 1 (OR: 1.20; 95% CI: 0.50–2.90) or 2 thresholds (OR: 1.88; 95% CI: 0.86–4.07).
Table 3.
Results for the associations of the compounding weakness thresholds on future cognitive impairment by obesity status
| No obesity | Obesity | |||
| OR | 95% CI | OR | 95% CI | |
| 1 Weakness category | 1.17 | 0.88, 1.55 | 1.20 | 0.50, 2.90 |
| 2 Weakness categories | 1.20 | 0.89, 1.61 | 1.88 | 0.86, 4.07 |
| 3 Weakness categories | 1.32 | 1.00, 1.74 | 2.76 | 1.29, 5.93 |
Reference: 0 weakness categories. CI, confidence interval; OR, odds ratio. The model controlled for cognitive impairment at current wave, follow-up years, sex, race, multimorbidity, age, smoking status, social engagement, self-rated health, depressive symptoms, moderate-to-vigorous physical activity participation, basic self-care limitation, and educational attainment.
DISCUSSION
The primary results of our study in which we performed a secondary analysis of HRS data suggest that older Americans falling below certain weakness thresholds were at risk for future cognitive impairment. Persons living without obesity but had HGS under the absolute weakness threshold had 54% greater odds for future cognitive impairment. Alternatively, older Americans with obesity and beneath weakness thresholds had greater odds for future cognitive impairment: 89% for absolute, 75% for BMI normalized, and 117% for body mass normalized, respectively. While those without obesity had 32% greater odds for future cognitive impairment when categorized as being beneath all 3-weakness thresholds, persons with obesity had 176% greater odds. Our findings indicate that the odds of cognitive impairment elevate when weakness and obesity are present, and that absolute and body size normalized thresholds (BMI and body mass) may yield differential estimates for future cognitive impairment when additionally classified by obesity status.
Although body size and strength capacity are linked, persons with obesity may challenge how strength is normalized to body size [8], which in turn, may influence associations with clinically-relevant health outcomes such as cognitive impairment. Our results indicate that while absolute weakness was linked to future cognitive impairment regardless of obesity status, persons with obesity also had greater odds for future cognitive impairment when beneath the BMI and body mass normalized weakness thresholds. Such findings suggest that obesity status might factor into how strength capacity is normalized for future cognitive impairment risk. When utilizing HGS to screen for future cognitive impairment, there should be acknowledgement for how normalizing HGS by body mass and BMI with respect to obesity status may influence these measures.
Physical activity participation may help in the prevention and treatment of AD/ADRD [27, 28]. While engagement in higher intensity physical activity lowered AD/ADRD risk, leisure activities are also inversely associated with a decreased risk of AD/ADRD. Indeed, physical activity participation is linked to lower AD/ADRD risk, but physical activity likewise helps in mitigating obesity and preserving muscle strength [29], and may have implications for cognitive function earlier in life [30]. Moreover, physical activity combats obesity [31], which in turn, is linked to executive function and dementia [32]. As such, physical activity could be particularly important for the triangulation between strength capacity, obesity status, and cognitive function.
The SDOC weakness cut-points present unique opportunities to incorporate all of these thresholds into a composite weakness measurement [7]. Previous research has indicated that the sensitivity for future cognitive impairment increased when persons are under all SDOC weakness thresholds [9]. False positives and negatives of an assessment for health can pose many problems, and could be explained by several factors. Our investigation showed that although older Americans below all the weakness thresholds had greater odds for future cognitive impairment regardless of obesity status, the odds were especially high for persons with obesity. These findings note that obesity is a factor when considering weakness status for cognitive function, particularly when normalizing HGS by body mass and BMI. Both weakness and obesity status should be considered when examining motor tasks for cognitive function during aging, and how strength capacity and body size may change over time.
Some limitations should be discussed. We performed analyses for weakness and future cognitive impairment by obesity status; however, comparing estimates and CIs between such statuses should be interpreted with the acknowledgement that such estimates are not being directly compared in our GEEs (i.e., reference group). Proxy respondents for participants with severe cognitive impairment may not have had their HGS collected. A low number of cases within our categorical variables challenged our ability to stratify the analyses by sociodemographic characteristics. Other cut-points for categorizing cognitive impairment may exist. Apolipoprotein E4 was not included in the publicly available RAND HRS dataset. More comprehensive cognitive assessments may have strengthened the predictive value of HGS and obesity.
Conclusions
The findings from this investigation indicate that older Americans beneath the weakness threshold for absolute HGS had greater odds for future cognitive impairment regardless of obesity status, but persons with obesity similarly had greater future cognitive impairment odds if they were beneath the BMI and body mass normalized weakness thresholds. Older Americans with obesity were especially at greater odds for future cognitive impairment when underneath all the weakness thresholds. Our findings suggest that weakness and obesity are associated with cognitive impairment, and caution should be noted for how HGS normalization procedures influence such measures and their predictive value. Continuing to examine the role of body size on HGS for health may yield additional insights.
AUTHOR CONTRIBUTIONS
Joshua Batesole (Investigation; Writing – original draft); Grant Tomkinson (Investigation; Writing – review & editing); Kirk Erickson (Investigation; Writing – review & editing); Donald Jurivich (Investigation; Writing – review & editing); Justin Lang (Investigation; Writing – review & editing); Brenda McGrath (Formal analysis; Investigation; Writing – review & editing); Sheria Robinson-Lane (Investigation; Writing – review & editing); Ashleigh Smith (Investigation; Writing – review & editing); Ryan McGrath (Conceptualization; Formal analysis; Investigation; Project administration; Visualization; Writing – original draft).
Supplementary Material
ACKNOWLEDGMENTS
The authors have no acknowledgements to report.
SUPPLEMENTARY MATERIAL
The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/ADR-230190.
FUNDING
Research reported in this publication was supported by the National Institute on Aging of the National Institutes of Health under Award Number R15AG072348 (to RM). The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health. AES is funded by a Dementia Australia Research Foundation Mid-Career Fellowship.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
DATA AVAILABILITY
Health and Retirement Study data are publicly available at their website.
REFERENCES
- [1]. Hale JM, Schneider DC, Mehta NK, Myrskylä M (2020) Cognitive impairment in the US: Lifetime risk, age at onset, and years impaired. SSM-Popul Health 11, 100577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Alzheimer’s Disease Facts and Figures. https://www.alz.org/alzheimers-dementia/facts-figures. Accessed on 30 November 2023.
- [3]. Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, Brayne C, Burns A, Cohen-Mansfield J, Cooper C, Costafreda SG, Dias A, Fox N, Gitlin LN, Howard R, Kales HC, Kivimäki M, Larson EB, Ogunniyi A, Orgeta V, Ritchie K, Rockwood K, Sampson EL, Samus Q, Schneider LS, Selbæk G, Teri L, Mukadam N (2020) Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 396, 413–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Lloret A, Monllor P, Esteve D, Cervera-Ferri A, Lloret A (2019) Obesity as a risk factor for Alzheimer’s disease: Implication of leptin and glutamate. Front Neurosci 13, 508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. Shaughnessy KA, Hackney KJ, Clark BC, Kraemer WJ, Terbizan DJ, Bailey RR, McGrath R (2020) A narrative review of handgrip strength and cognitive functioning: Bringing a new characteristic to muscle memory. J Alzheimers Dis 73, 1265–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]. Manini TM, Patel SM, Newman AB, Travison TG, Kiel DP, Shardell MD, Pencina KM, Wilson KE, Kelly TL, Massaro JM, Fielding RA, Magaziner J, Correa-de-Araujo R, Kwok TCY, Hirani V, Karlsson MK, D’Agostino RB Sr, Mellström D, Ohlsson C, Ribom E, Jordan JM, Bhasin S, Cawthon PM (2020) Identification of sarcopenia components that discriminate slow walking speed: A pooled data analysis. J Am Geriatr Soc 68, 1419–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]. Cawthon PM, Manini T, Patel SM, Newman A, Travison T, Kiel DP, Santanasto AJ, Ensrud KE, Xue Q-L, Shardell M, Duchowny K, Erlandson KM, Pencina KM, Fielding RA, Magaziner J, Kwok T, Karlsson M, Ohlsson C, Mellström D, Hirani V, Ribom E, Correa-de-Araujo R, Bhasin S (2020) Putative cut-points in sarcopenia components and incident adverse health outcomes: An SDOC analysis. J Am Geriatr Soc 68, 1429–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. McGrath R (2019) Comparing absolute handgrip strength and handgrip strength normalized to body weight in aging adults. Aging Clin Exp Res 31, 1851–1853. [DOI] [PubMed] [Google Scholar]
- [9]. McGrath R, Tomkinson GR, Hamm JM, Juhl K, Knoll K, Parker K, Smith AE, Rhee Y (2023) The role of different weakness cut-points for future cognitive impairment in older Americans. J Am Med Dir Assoc 24, 1936–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. McGrath R, Jurivich DA, Christensen BK, Choi B-J, Langford M, Rhee Y, Tomkinson GR, Hackney KJ (2023) Examining the role of different weakness categories for mobility and future falls in older Americans. Aging Clin Exp Res 35, 2491–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11]. Patel SM, Duchowny KA, Kiel DP, Correa-de-Araujo R, Fielding RA, Travison T, Magaziner J, Manini T, Xue Q, Newman AB (2020) Sarcopenia definition & outcomes consortium defined low grip strength in two cross-sectional, population-based cohorts. J Am Geriatr Soc 68, 1438–1444. [DOI] [PubMed] [Google Scholar]
- [12]. Sonnega A, Faul JD, Ofstedal MB, Langa KM, Phillips JW, Weir DR (2014) Cohort Profile: The Health and Retirement Study (HRS). Int J Epidemiol 43, 576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. Fisher GG, Ryan LH (2018) Overview of the Health and Retirement Study and Introduction to the Special Issue. Work Aging Retire 4, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sample Sizes and Response Rates. Health and Retirement Study. https://hrs.isr.umich.edu/sites/default/files/biblio/sampleresponse.pdf. Accessed on 30 November 2023.
- [15].HRS Data Book. https://hrs.isr.umich.edu/about/data-book. Accessed on 30 November 2023.
- [16]. Plassman BL, Newman TT, Welsch KA, Helms M, Breitner J (1994) Properties of the telephone interview for cognitive status: Application in epidemiological and longitudinal studies. Cogn Behav Neurol 7, 235–241. [Google Scholar]
- [17]. Hale JM, Bijlsma MJ, Lorenti A (2021) Does postponing retirement affect cognitive function? A counterfactual experiment to disentangle life course risk factors. SSM-Popul Health 15, 100855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18]. Fong TG, Fearing MA, Jones RN, Shi P, Marcantonio ER, Rudolph JL, Yang FM, Kiely DK, Inouye SK (2009) Telephone interview for cognitive status: Creating a crosswalk with the Mini-Mental State Examination. Alzheimers Dement 5, 492–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]. Langa KM, Larson EB, Karlawish JH, Cutler DM, Kabeto MU, Kim SY, Rosen A (2008) Trends in the prevalence and mortality of cognitive impairment in the United States: Is there evidence of a compression of cognitive morbidity? Alzheimers Dement 4, 134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20]. Crimmins E, Guyer H, Langa K, Ofstedal MB, Wallace R, Weir D (2008) Documentation of Physical Measures, Anthropometrics and Blood Pressure in the Health and Retirement Study. https://hrs.isr.umich.edu/sites/default/files/biblio/dr-011.pdf. Accessed 30 November 2023.
- [21]. Ekstrand E, Lexell J, Brogårdh C (2016) Grip strength is a representative measure of muscle weakness in the upper extremity after stroke. Top Stroke Rehabil 23, 400–405. [DOI] [PubMed] [Google Scholar]
- [22]. McGrath R, Cawthon P, Clark B, Fielding R, Lang J, Tomkinson G (2022) Recommendations for reducing heterogeneity in handgrip strength protocols. J Frailty Aging 11, 143–150. [DOI] [PubMed] [Google Scholar]
- [23].Centers for Disease Control and Prevention. Adult Obesity Facts. https://www.cdc.gov/obesity/data/adult.html. Accessed on 30 November 2023.
- [24]. Turvey CL, Wallace RB, Herzog R (1999) A revised CES-D measure of depressive symptoms and a DSM-based measure of major depressive episodes in the elderly. Int Psychogeriatr 11, 139–148. [DOI] [PubMed] [Google Scholar]
- [25]. Feng X, Croteau K, Kolt GS, Astell-Burt T (2016) Does retirement mean more physical activity? A longitudinal study. BMC Public Health 16, 605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. Howrey B, Avila J, Downer B, Wong R (2020) Social engagement and cognitive function of older adults in Mexico and the United States. Innov Aging 4, 560–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27]. Erickson KI, Hillman C, Stillman CM, Ballard RM, Bloodgood B, Conroy DE, Macko R, Marquez DX, Petruzzello SJ, Powell KE (2019) Physical activity, cognition, and brain outcomes: A review of the 2018 physical activity guidelines. Med Sci Sports Exerc 51, 1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28]. De la Rosa A, Olaso-Gonzalez G, Arc-Chagnaud C, Millan F, Salvador-Pascual A, García-Lucerga C, Blasco-Lafarga C, Garcia-Dominguez E, Carretero A, Correas AG (2020) Physical exercise in the prevention and treatment of Alzheimer’s disease. J Sport Health Sci 9, 394–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29]. Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, George SM, Olson RD (2018) The physical activity guidelines for Americans. JAMA 320, 2020–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30]. Tait JL, Collyer TA, Gall SL, Magnussen CG, Venn AJ, Dwyer T, Fraser BJ, Moran C, Srikanth VK, Callisaya ML (2022) Longitudinal associations of childhood fitness and obesity profiles with midlife cognitive function: An Australian cohort study. J Sci Med Sport 25, 667–672. [DOI] [PubMed] [Google Scholar]
- [31]. Niemiro GM, Rewane A, Algotar AM (2023) Exercise and fitness effect on obesity. In StatPearls StatPearls Publishing, Treasure Island (FL). [PubMed]
- [32]. Quaye E, Galecki AT, Tilton N, Whitney R, Briceño EM, Elkind MS, Fitzpatrick AL, Gottesman RF, Griswold M, Gross AL (2023) Association of obesity with cognitive decline in black and white Americans. Neurology 100, e220–e231. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Health and Retirement Study data are publicly available at their website.