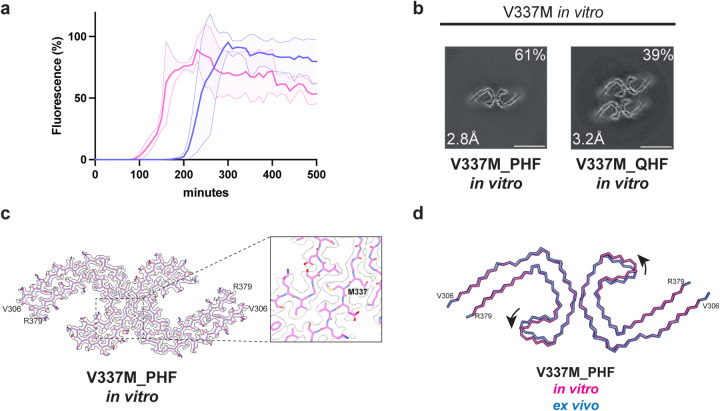
Figure 3. In vitro assembly of V337M tau(297–391).
a, In vitro assembly assay monitored by thioflavin T (ThT) fluorescence of V337M tau(297–391) (magenta) and wild-type tau(297–391) (blue).
b, Cross-sections through the cryo-EM reconstructions, perpendicular to the helical axis and with a projected thickness of approximately one rung, are shown for assembled V337M tau(297–391).
c, Cryo-EM density map and atomic model of paired helical filament (PHF). Two identical protofilaments extend from V306-R379. Inset: Zoomed-in view showing the mutant methionine at position 337.
d, Overlay of PHFs assembled from recombinant V337M tau(297–391) (magenta) and extracted from the frontal cortex of an individual with mutation V337M (blue). The rmsd between Cα atoms is 0.80 Å with a 9° rotation of the β-helix region relative to the rest of the ordered core being the main difference between the two structures.