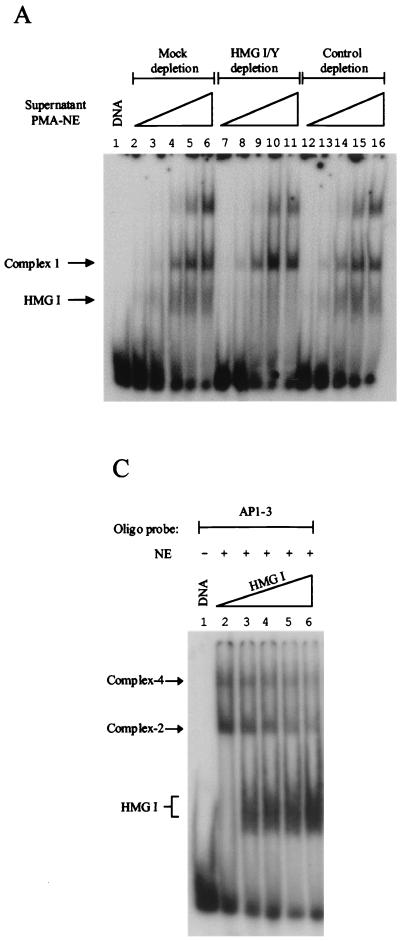
FIG. 6.
HMG I can both selectively inhibit and promote endogenous factor binding to site AP1-3. (A) The 60% (NH4)2SO4 supernatant fraction, derived from PMA-induced nuclear extracts (NE), was mock immunodepleted (lanes 2 to 6) or immunodepleted with affinity-purified antibodies against HMG I/Y (lanes 7 to 11) or H2A (control, lanes 12 to 16). A mobility gel shift assay, using site AP1-3, was carried out using these immunodepleted supernatant fractions. Lanes 2 to 6, 7 to 11, and 12 to 16 received 2, 5, 10, 15, and 20 μg of the supernatant fraction, respectively. (B) PMA-induced supernatant fractions that were immunodepleted with either control (H2A) or HMG I affinity-purified antibodies were titrated back to a PMA-induced nuclear precipitated fraction. Lanes 2 to 12 received 7 μg of nuclear (NH4)2SO4-precipitated proteins. Lanes 3 to 7 and lanes 8 to 12 received 5, 10, 20, 30, and 40 μg of H2A- or HMG I/Y-depleted supernatant fractions, respectively. (C) Increasing amounts of recombinant HMG I were added to DNA-binding reactions that received 10 μg of uninduced nuclear precipitated extract and a labeled oligonucleotide that contained site AP1-3. Lanes 2 to 6 received 0, 25, 50, 100, and 200 ng of HMG I, respectively.