Abstract
BACKGROUND
Patients with hemorrhagic shock and trauma (HS/T) are vulnerable to the endotheliopathy of trauma (EOT), characterized by vascular barrier dysfunction, inflammation, and coagulopathy. Cellular therapies such as mesenchymal stem cells (MSCs) and MSC extracellular vesicles (EVs) have been proposed as potential therapies targeting the EOT. In this study we investigated the effects of MSCs and MSC EVs on endothelial and epithelial barrier integrity in vitro and in vivo in a mouse model of HS/T. This study addresses systemic effects of HS/T on multiorgan EOT in HS/T model.
METHODS
In vitro, pulmonary endothelial cell (PEC) and Caco-2 intestinal epithelial cell monolayers were treated with control media, MSC conditioned media (CM), or MSC EVs in varying doses and subjected to a thrombin or hydrogen peroxide (H2O2) challenge, respectively. Monolayer permeability was evaluated with a cell impedance assay, and intercellular junction integrity was evaluated with immunofluorescent staining. In vivo, a mouse model of HS/T was used to evaluate the effects of lactated Ringer’s (LR), MSCs, and MSC EVs on endothelial and epithelial intercellular junctions in the lung and small intestine as well as on plasma inflammatory biomarkers.
RESULTS
MSC EVs and MSC CM attenuated permeability and preserved intercellular junctions of the PEC monolayer in vitro, whereas only MSC CM was protective of the Caco-2 epithelial monolayer. In vivo, both MSC EVs and MSCs mitigated the loss of endothelial adherens junctions in the lung and small intestine, though only MSCs had a protective effect on epithelial tight junctions in the lung. Several plasma biomarkers including MMP8 and VEGF were elevated in LR- and EV-treated but not MSC-treated mice.
CONCLUSIONS
In conclusion, MSC EVs could be a potential cell-free therapy targeting endotheliopathy after HS/T via preservation of the vascular endothelial barrier in multiple organs early after injury. Further research is needed to better understand the immunomodulatory effects of these products following HS/T and to move toward translating these therapies into clinical studies.
Keywords: mesenchymal stem cells, mesenchymal stem cell extracellular vesicles, hemorrhagic shock, trauma
BACKGROUND
Patients with severe injuries and hemorrhagic shock are susceptible to the endotheliopathy of trauma (EOT), which includes vascular barrier compromise, systemic inflammation, and dysfunctional coagulation.1–3 The EOT is closely linked with multiple organ failure and contributes to increased mortality after injury.4,5 While plasma-based resuscitation and modern trauma systems have improved outcomes in severely injured patients,6,7 trauma remains a leading cause of death in the United States,8 and novel therapies are needed.
Bone marrow derived mesenchymal stem cells (MSCs) are known to have vasculoprotective, anti-inflammatory, and regenerative properties and are the focus of numerous ongoing clinical trials.9,10In light of these properties, MSCs have been proposed as a potential therapy targeting the EOT and have been shown to reduce injury in multiple organs11–14 and reverse bone marrow suppression15 in rodent models of hemorrhagic shock and trauma (HS/T). Though initially MSCs were thought to act through their ability to home to injured tissue, the majority of intravenously administered MSCs become trapped in capillary networks such as in the lungs; one of their primary mechanisms of action is now understood to be through the release of bioactive soluble factors and extracellular vesicles (EVs).10,16,17 EVs are membrane-bound particles containing RNA, DNA, protein, and lipids from their parent cells and are highly effective mediators of intercellular communication.18 MSC EVs have been proposed as a “cell-free” therapy as they have been shown to recapitulate the therapeutic properties of MSCs.17,19
We recently demonstrated in a mouse model of HS/T that both MSCs and MSC EVs mitigate histologic injury and vascular permeability to a 10 kDa dextran dye in the small intestine and lungs.12 The purpose of the current study was to further investigate the molecular mechanism of MSCs and MSC EVs in HS/T, specifically their effects on endothelial and epithelial barrier integrity and on systemic inflammatory biomarkers. We hypothesized that MSCs and MSC EVs would (1) preserve pulmonary endothelial and intestinal epithelial barrier integrity in vitro and (2) preserve intercellular junctions in the small intestine and lungs and reduce plasma inflammatory biomarker levels in vivo.
METHODS
Mesenchymal Stem Cell Culture and EV Isolation
Human bone marrow-derived MSCs (passage 1) were obtained from Rooster Bio Inc. (Frederick, MD) and expanded initially on a Terumo Quantum Device (Terumo, Lakewood, CO) to generate passage 2 cells that were used in all studies. MSCs were grown in Mesenchymal Stem Cell Growth Medium 2 (PromoCell, Heidelberg, Germany) and maintained at 37°C and 5% CO2 in a humidified incubator. To isolate MSC EVs, the MSCs were grown to 80% confluence, then serum-starved for 48 hours. The MSC conditioned media (CM) was collected and centrifuged at 1500g × 10 minutes to remove cellular debris, then filtered using a 0.22μm filter. EVs were isolated from the MSC CM using a Tangential Flow Filtration System with the Pellicon® XL50 Cassette with Biomax® 500 kDa Membrane (MilliporeSigma, Burlington, MA). A subset of the isolated EVs were further concentrated using an Amicon Ultra-2 Centrifugal Filter 3K Device (MilliporeSigma) according to manufacturer instructions for use in the in vitro experiments. Aliquots of isolated EVs were stored at −80°C. The MSC EVs used in this study were previously characterized by flow cytometry, nanoparticle tracking analysis, and spectrophotometry as previously described,12 and scanning electron microscopy images were also captured for this study (Supplemental Figure 1).
Pulmonary Endothelial Cell (PEC) and Caco-2 Intestinal Epithelial Cell Culture
Human pulmonary microvascular endothelial cells (PECs) were obtained from PromoCell and grown in Endothelial Cell Growth Medium MV2 (PromoCell). Passages 3–7 were used in all experiments. Caco-2 human intestinal epithelial cells were obtained from American Type Culture Collection (ATCC, Manassas, VA) and were grown in Eagle’s Minimum Essential Medium (ATCC) supplemented with 20% Fetal Bovine Serum and 1% penicillin/streptomycin. Passages 2–5 were used in all experiments. Both cell lines were maintained at 37°C and 5% CO2 in a humidified incubator
In Vitro Pulmonary Endothelial Cell (PEC) Monolayer Barrier Integrity and Intercellular Junction Immunostaining
Pulmonary endothelial cell (PEC) monolayer barrier integrity was measured in vitro using an electric cell-substrate impedance sensing system (ECIS 1600, Applied BioPhysics, Troy, NY). To evaluate the PEC monolayer, PECs (50,000 cells per well) were seeded onto a 96-well plate containing electrodes that had been pre-treated with L-cysteine and were grown to confluence. The PEC monolayers were serum-starved for one hour and once resistances had stabilized were then pre-treated with MSC control media (10% or 20% v/v), MSC CM (10% or 20% v/v), or MSC EVs (10 or 30 μg/ml). After 30 minutes the PEC monolayers were challenged with thrombin at 0.2u/ml to induce paracellular permeability. Resistances were measured in 5-minute intervals at 4000 Hz. Data were normalized to the mean resistance of the monolayers before the treatments. Resistance tracings and area under the curve (AUC) plots were generated for each treatment group to compare monolayer integrity.
To evaluate intercellular junction integrity of the PEC monolayer, PECs were separately grown on cover slips (coated with collagen type 1) in 24-well plates (50,000 cells per well). The wells were pre-treated with MSC control media (10% or 20% v/v), MSC CM (10% or 20% v/v) or MSC EVs (30 μg/ml) for 30 minutes then challenged with thrombin 0.2u/ml. 5 min after the addition of thrombin, the cells were washed three times with PBS then fixed with 4% PFA. Immunostaining was then performed using antibodies against VE-cadherin (Cell Signaling, Danvers, MA) and Zonula Occludens-1 (ZO-1, Abcam, Burlingame, CA). F-actin was detected with Texas Red Phalloidin (Cell Signaling). Representative images were captured using a Nikon Eclipse 80i microscope (Nikon, Melville, NY) with an RT-scmos camera (SPOT Imaging, Sterling Heights, MI).
In Vitro Caco-2 Intestinal Epithelial Cell Monolayer Barrier Integrity and Intercellular Junction Immunostaining
To evaluate the Caco-2 intestinal epithelial monolayer, Caco-2 cells (25,000 cells per well) were seeded onto a 96-well plate for ECIS as above and grown to confluence. The Caco-2 monolayers were serum-starved for 2 hours and once resistances had stabilized were then pre-treated with MSC control media (10% or 25% v/v), MSC CM (10% or 25% v/v), or MSC EVs (10, 30, or 50 μg/ml). After 30 minutes the Caco-2 cell monolayers were challenged with hydrogen peroxide (H2O2) at 2.5mM to cause oxidative stress; this dose was chosen based on preliminary studies in the lab showing decreased resistance across the monolayer in this model. Resistances were measured in 4-minute intervals at 1000 Hz. Data normalization, resistance tracings, and AUC plot generation were conducted as above.
To evaluate intercellular junction integrity of the Caco-2 monolayer, Caco-2 cells were separately seeded on to 24-well plates (80,000 cells per well). The cells were pre-treated with MSC control media (10% or 25% v/v), MSC CM (10% or 25% v/v) or MSC EVs (30 μg/ml) for 30 minutes then challenged with H2O2 2.5mM. 2.5 hours after exposure to H2O2, the cells were washed three times with PBS then fixed with 4% PFA. Immunostaining was then performed using antibody against ZO-1 (Abcam), and F-actin was detected using Texas Red Phalloidin (Cell Signaling). Representative images were captured using a Revolve microscope (Echo Inc., San Diego, CA).
Animal Studies
Animal studies were performed with approval of the Institutional Animal Care and Use Committee (IACUC) at UCSF. The experiments were conducted in compliance with the ARRIVE guidelines for animal models and the National Institutes of Health (NIH) guidelines on the use of laboratory animals. All animals were house in a room with access to food and water ad libitum, controlled temperature, and 12:12-hour light-dark cycles.
Mouse Model of Hemorrhagic Shock and Trauma
Male C57BL6 mice, 8–12 weeks old, were obtained from The Jackson Laboratory (Sacramento, CA) (N=20 total). Mice underwent an established model of HS/T.12,20,21 Briefly, the mice were anesthetized with isoflurane and maintained at a body temperature between 35°C and 37°C using a heating plank. The bilateral femoral arteries were cannulated with heparinized catheters, one for continuous blood pressure monitoring (PowerLab 7, AD Instruments, Dunedin, New Zealand), and the other for blood withdrawal and resuscitation. A 2cm midline laparotomy was also performed to induce additional trauma. Mice were subsequently bled to a mean arterial pressure (MAP) of 35mmHg for 90 minutes and then resuscitated with a 200μL fluid bolus containing 1) lactated Ringer’s (LR), 2) 1 × 106 MSCs in LR, or 3) MSC EVs (30μg) in PBS. These doses were chosen based on previous work demonstrating efficacy of MSC and MSC EVs in this model.12,21 Sham mice underwent cannulation without laparotomy or hemorrhage. Mice were monitored hemodynamically for an additional 30 minutes after resuscitation. Two hours post-resuscitation, the mice were re-anesthetized with isoflurane. Blood was collected via cardiac puncture and the mice were perfused with 10mL of ice-cold PBS. Sodium citrate 3.2% was added to the blood in a 1:9 ratio prior to centrifugation at 3000g for 10 minutes to isolate the plasma fraction, which was stored at −80°C. The lungs and a segment of small intestine were harvested and flash-frozen in isopentane and stored at −80°C.
Intercellular Junction Immunostaining of the Small Intestine and Lungs
The lungs and small intestine from N=5 mice per group were sectioned at 10μm thickness. The sections were fixed in ice-cold 95% EtOH for 20 minutes then 100% acetone for 1 minutes. Immunostaining was then performed using antibodies against the adherens junction protein VE-cadherin (R&D Systems, Minneapolis, MN) and the tight junction proteins ZO-1 (Abcam, Burlingame, CA) and claudin-4 (Thermo Fisher, Waltham, MA). Sections were imaged in a blinded fashion with a Nikon Eclipse 80i microscope (Nikon) with an RT-scmos camera (SPOT Imaging), and representative images were selected from each animal for qualitative comparison.
Plasma Inflammatory Biomarker Analysis
Mouse plasma samples (N=5 per group) were analyzed using a custom multiplex Luminex® Discovery Assay Kit (R&D Systems, Minneapolis, MN) according to the manufacturer’s protocol. The following analytes were included: Angiopoietin-2 (Ang-2), C-X-C Motif Chemokine Ligand 10 (CXCL10), CXCL12, Intercellular Adhesion Molecule-1 (ICAM-1), Interferon gamma (IFN-γ), Interleukin-1beta (IL-1β), IL-2, IL-4, IL-6, IL-6 receptor alpha (IL-6Ra), IL-10, Matrix Metalloproteinase-8 (MMP8), MMP9, Syndecan-1, Tumor Necrosis Factor alpha (TNFα), TNF Receptor Superfamily Member 1a (TNFRSF1a), TNFRSF1b, TNF Superfamily Member 13b (TNFSF13b), and Vascular Endothelial Growth Factor (VEGF). Plasma samples were thawed and centrifuged at 16,000g for 4 min prior to use. Samples were then diluted 1:2 and 1:10 in appropriate diluent and pipetted onto a 96-well plate, mixed with magnetic beads coated with antibodies, and incubated for 2 hrs at room temperature on a horizontal orbital microplate shaker at 800rpm. Three washes with wash buffer were performed, then the beads were incubated with the biotinylated antibody cocktail for 1 hr at room temperature on the orbital shaker at 800rpm. After another three washes, the beads were incubated with streptavidin-PE for 30 minutes on the shaker. The beads were washed a final three times then resuspended in wash buffer prior to being read on a MAGPIX System (Luminex Corp., Austin, TX). xPONENT 4.2 software (Luminex Corp.) was used for data acquisition.
Statistical Analysis
Area under the curve values for the in vitro experiments were compared using one-way ANOVA with Tukey’s post hoc tests. Mean arterial pressures (MAPs) were compared using repeated measures two-way ANOVA with Tukey’s multiple comparisons test. For the plasma biomarker analysis, first outliers were removed using the ROUT method in Prism 9.0 (GraphPad Inc., San Diego, CA) and data were assessed for normality using the Shapiro-Wilk test. One-way ANOVA with post-hoc Tukey’s test was used for normally distributed data, and Kruskal-Wallis testing with Dunn’s multiple comparisons test was used for data that did not pass the normality testing. P<.05 was considered significant. All analyses were performed using Prism 9.0. Data in this manuscript are presented as mean ± SD.
RESULTS
MSC EV Characterization
The MSC EVs used in this study have been characterized previously by nanoparticle tracking analysis, spectrophotometry, and flow cytometry.12 The EVs had a concentration of 1.16 ×109 particles/ml with a size peak at 123 nm and protein content of 160 μg/ml. A subset of the EVs further concentrated for the EV experiments had a protein content of 330 μg/ml. By flow cytometry the EVs were positive for EV-specific markers CD69, CD81 and CD9, MSC markers CD73 and CD90, and negative for negative control markers CD31, CD45, and HLA-DR. In addition, images of the MSC EVs via scanning electron microscopy were obtained, consistent with the particle size demonstrated via nanoparticle tracking analysis (Supplemental Figure 1).
MSC Conditioned Media and MSC EVs Protect the Pulmonary Endothelial Barrier in Vitro
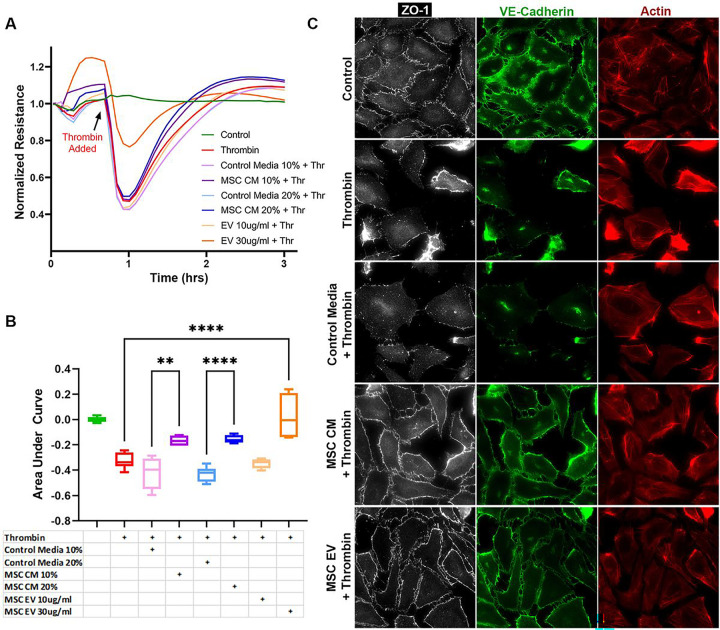
Resistance tracings generated by ECIS and AUC calculations demonstrated a drop in resistance across PEC monolayers exposed to thrombin, indicating increased paracellular permeability (Figure 1A, 1B). MSC CM refers to cell culture media containing the MSC secretome, whereas MSC control media has not been exposed to MSCs and thus does not contain MSC-secreted factors or EVs. Cell monolayers that had been pre-treated with MSC CM had significantly higher resistances than those treated with MSC control media at both of the tested doses. The higher dose of MSC EVs at 30 μg/ml was also protective.
Figure 1. Effects of MSC CM and MSC EVs on a Pulmonary Endothelial Cell Monolayer in Vitro.
(A)Pulmonary endothelial cell (PEC) monolayer resistance tracings at 4000Hz in response to thrombin challenge. N=4 cell replicates per group. (B) Area under the curve (AUC) plot for resistances following the addition of thrombin. **p<.01, ****p<.0001 by one-way ANOVA with post-hoc Tukey’s tests. (C)Representative images (40x magnification) of immunofluorescence staining of PEC monolayers for Zonula Occludens-1 (ZO-1, white), VE-cadherin (green), and Actin (red). The addition of thrombin caused decreased resistance across the monolayer as well as loss of ZO-1 and VE-cadherin staining and gap formation with cells. Pre-treatment of the cell monolayers with MSC CM (20%) or MSC EVs (30μg/ml) partially attenuated these effects, whereas MSC control media (20%) was not protective.
Consistent with the ECIS tracings, immunostaining for the tight junction protein ZO-1 and adherens junction protein VE-cadherin demonstrated a loss of ZO-1 and VE-cadherin staining among PEC monolayers exposed to thrombin (Figure 1C). In addition, thrombin exposure resulted in gap formation or separation between cells. Pre-treatment with MSC CM or MSC EVs resulted in relative preservation of PEC barrier integrity as evidenced by attenuation of the loss of ZO-1 and VE-cadherin staining and decreased gap formation. F-actin staining of the PEC monolayer showed reduced stress fiber activation, when compared to the thrombin challenged group.
MSC Conditioned Media but not MSC EVs Protect the Intestinal Epithelial Barrier in Vitro
Caco-2 intestinal epithelial cell monolayers exhibited decreased resistance or increased permeability in response to H2O2 exposure (Figure 2A, 2B). MSC CM was significantly protective compared to control media at the higher dose (25%) tested. However, MSC EV pre-treatment was not protective at any of the tested doses. Immunostaining was also performed on the Caco-2 monolayers to evaluate ZO-1 and actin (Figure 2C). H2O2 exposure resulted in re-distribution of actin filaments and decreased ZO-1 staining compared to control conditions. Pre-treatment with MSC CM preserved actin organization and attenuated the loss of ZO-1. However, similar to the results demonstrated by ECIS, MSC EV pre-treatment did not affect ZO-1 or actin staining.
Figure 2. Effects of MSC CM and MSC EVs on the Caco-2 Intestinal Epithelial Cell Monolayer in Vitro.
(A) Caco-2 intestinal epithelial cell resistance tracings at 1000Hz in response to H2O2 challenge. N=4 cell replicates per group. (B) Area under the curve (AUC) plot for resistances following the addition of H2O2. ***p<.001 by one-way ANOVA with post-hoc Tukey’s tests. (C) Representative images (10x magnification) of immunofluorescence staining of Caco-2 monolayers for Zonula Occludens-1 (ZO-1, green), Actin (red), and DAPI (blue). The addition of H2O2 caused decreased resistance across the monolayer as well as relative loss of ZO-1 staining and actin redistribution. MSC CM (25%) pre-treatment decreased the effects of H2O2, whereas treatment with MSC control media (25%) or MSC EVs (30 μg/ml) were not protective.
MSCs and MSC EVs Preserve Endothelial Adherens Junctions in the Intestine and Lung in Vivo
The HS/T model schematic is depicted in Figure 3. Mean arterial pressures (MAPs) were similar among mice resuscitated with LR, MSCs, or MSC EVs. Sections of the lungs and small intestine of mice subjected to HS/T were stained to evaluate endothelial and epithelial barrier integrity in vivo. In the lungs, HS/T with LR resuscitation induced a qualitative loss of VE-cadherin (adherens junction) staining among endothelial-lined blood vessels and alveolar capillaries compared to sham mice. MSC and MSC EV treatment resulted in relative preservation of VE-cadherin staining. Moreover, there was notable compromise of ZO-1 (tight junction) staining in the epithelial-lined airways that underwent HS/T compared to sham mice, however this finding was partially mitigated in the MSC group. There was also loss of ZO-1 staining in the alveoli regardless of treatment group after HS/T (Figure 4). In the small intestine, sections stained for VE-cadherin demonstrated a loss of VE-cadherin staining in the submucosal vessels after HS/T in LR-treated mice, whereas MSC and MSC EV treatment qualitatively preserved VE-cadherin staining in these vessels. Staining for ZO-1 and claudin-4 was also performed to evaluate epithelial integrity, however, there were no substantial differences across the sham or injured groups (Figure 5).
Figure 3. Hemorrhagic Shock and Trauma Mouse Model.
(A) Depiction of the 90-minute HS/T model. Mice were resuscitated with LR, MSCs, or MSC EVs (N=5 mice per group). Blood collection and organ harvest were performed 2 hours after the end of shock. (B) Mean arterial pressures (MAPs) during the 30 minutes post-resuscitation. There was no effect of resuscitation group on MAPs (p=0.74) by two-way ANOVA with repeated measures.
Figure 4. Intercellular Junction Staining in the Lung after Hemorrhagic Shock and Trauma (HS/T).
Representative images (20x magnification) of lungs stained for VE-cadherin (endothelial adherens junctions; red), ZO-1 (tight junctions; green), and DAPI (nuclei; blue). Mice subjected to HS/T and resuscitated with LR demonstrate loss of ZO-1 staining between epithelial cells in the airways as well as in the alveolar epithelium. LR-resuscitated mice also demonstrate a loss of staining for VE-cadherin in small blood vessels. Treatment with MSCs or MSC EVs restored VE-cadherin staining suggesting a protective effect on endothelial barrier integrity. MSC treatment also partially protected against loss of ZO-1 in the epithelial-lined airways. Open arrow points to airway. Closed arrowpoints to blood vessel. Yellow arrow points to alveoli containing capillaries and epithelial cells.
Figure 5. Intercellular Junction Staining in the Small Intestine after Hemorrhagic Shock and Trauma (HS/T).
Representative images of small intestine stained for VE-cadherin (adherens junctions; red; 10x magnification), ZO-1 (tight junctions; green; 20x magnification), claudin-4 (tight junctions; red; 20x magnification), and DAPI (nuclei; blue). LR-treated mice demonstrated a loss of VE-cadherin in the submucosal blood vessels (see 40x image inset) of the small intestine, whereas MSC or MSC EV treatment attenuated this finding. Epithelial barrier integrity was evaluated with ZO-1 and claudin-4 staining, however no differences were evident between groups.
Plasma Biomarker Analysis
The levels of each plasma inflammatory biomarker are listed in Table 1. Biomarkers that showed significant differences between any of the groups are depicted in Figure 6. Compared to sham mice, LR-treated mice had significantly higher levels of IL-10, MMP-8, MMP-9, TNFRSF1b, and VEGF, MSC-treated mice had higher levels of TNFRSF1b and showed a trend toward higher levels of CXCL12 (p=0.06), and EV-treated mice had higher levels of IFN-y, IL-1B, IL-6, IL-10, MMP-8, MMP-9, TNFa, TNFRSF1b, and VEGF and showed a trend toward higher levels of CXCL10 (p=0.06) and CXCL12 (p=0.06). Other biomarkers were similar across groups. Notably, both MMP-8 and VEGF were significantly elevated among the LR- and EV-treated groups compared to sham mice, whereas these biomarker levels were not significantly different between MSC-treated mice and sham mice.
Table 1.
Plasma Inflammatory Biomarkers after Hemorrhagic Shock and Trauma.
| Analyte | Sham (pg/ml) | LR (pg/ml) | MSC (pg/ml) | EV (pg/ml) |
|---|---|---|---|---|
| Ang2 | 27324±3424 | 23371±3809 | 30293±6566 | 24191±5852 |
| CXCL10 | 0±0 | 7379±5459 | 16318±18089 | 28938±23111 |
| CXCL12 | 1472±85.73 | 1612±75.6 | 1687±154a | 1687±124.7a |
| ICAM-1 | 15294±4666 | 12057±3535 | 12936±2642 | 14130±2570 |
| IFN-γ | 0±0 | 2.68±3.627 | 0±0 | 5.46±5.26a,b |
| IL-1β | 0±0 | 104.4±88.06 | 61.4±104 | 151.6±130a |
| IL2 | 0±0 | 0.6±1.34 | 0.72±1.61 | 2.04±2.82 |
| IL4 | 330±7.97 | 324.8±27.58 | 323.8±36.41 | 331±23.01 |
| IL6 | 53.2±35.32 | 10198±9560 | 11992±16983 | 60396±41279a |
| IL6Ra | 10349±909.3 | 10780±639 | 11144±1209 | 10399±1529 |
| IL10 | 3.6±0.55 | 857.4±461.1a | 299±259.8 | 1229±895.8a |
| MMP8 | 282150±103143 | 4577903±1649162a | 3615807±2199024 | 8471390±3408584a,b |
| MMP9 | 10488±4969 | 118897±35588a | 94408±51062a | 146319±59425a |
| Syndecan-1 | 8031±3599 | 9799±1811 | 12253±3066 | 10605±2461 |
| TNF-α | 1.8±0.45 | 84.4±52.34 | 60.8±59.78 | 189.8±126.3a |
| TNFRSFIa | 1075±272.6 | 5002±3029 | 4065±2327 | 5060±3407 |
| TNFRSFIb | 3347±854.7 | 14819±3996a | 14213±5198a | 19580±3965a |
| TNFRSF3b | 5920±978 | 5506±802 | 6253±1194 | 7120±2088 |
| VEGF | 17.6±4.34 | 144.2±82.35a | 116.2±84.53 | 247.8±57.9a,b |
Luminex multiplex measurement of plasma from mice at 2 hours post-resuscitation. Groups were compared using one-way ANOVA for normally distributed data and Kruskal-Wallis testing for data that were not normally distributed. Outliers were defined by the GraphPad program and were removed from analysis. Numbers are mean±SD, N=5 mice per group.
Statistically significant difference compared to sham
Statistically significant difference compared to MSC. P values as indicated in Figure 6.
Figure 6. Plasma Biomarker Analysis.
Plasma biomarkers of inflammation and endothelial function were evaluated using a custom Luminex® assay (N=5 mice per group). Only biomarkers with significant differences between groups are shown here. Groups were compared using one-way ANOVA for normally distributed data and Kruskal-Wallis testing for data that were not normally distributed. Outliers were defined by the GraphPad program and were removed from analysis. Measurements are expressed in pg/ml.
CONCLUSIONS
Our previous work has shown that MSC EVs recapitulate the ability of MSCs to mitigate histologic injury and vascular permeability in the lungs and small intestine in mice after HS/T. This study further demonstrates that MSCs and MSC EVs may target the EOT via preservation of the vascular endothelial barrier early after injury.
In this study, we demonstrated in vitro that both MSCs and MSC EVs preserve barrier properties of a pulmonary endothelial monolayer. While MSC CM also helped to maintain barrier integrity of a Caco-2 intestinal epithelial monolayer, MSC EVs were not protective at multiple tested doses. This finding may be due to higher concentrations of protective factors or additional soluble factors in the MSC CM compared to the MSC EVs. In vivo in a mouse model of HS/T, both MSCs and MSC EVs attenuated the loss of endothelial adherens junction staining in blood vessels in the lung and small intestine. MSCs but not MSC EVs resulted in a partial protective effect on epithelial tight junctions in the lung. Epithelial tight junctions in the small intestine were not clearly affected in this model. Altogether these results suggest that one potential therapeutic mechanism of MSCs and MSC EVs in HS/T is an early protective effect on vascular barrier integrity.
In addition, at two hours post resuscitation, we did find that there were several differences in the inflammatory biomarker profiles between groups. For example, both MMP8 and VEGF were significantly elevated among LR- and EV-treated (but not MSC-treated) mice compared to sham. MMP8 is known to decreased expression of tight junction proteins in endothelial cells;22 and VEGF is a potent vascular permeabilizing agent via multiple mechanisms including phosphorylation and loss of tight and adherens junctions, induction of matrix metalloproteinase expression, and negative regulation of pericyte function.23 This suggests that there may be some differences in the immunomodulatory effects of MSCs and MSC EVs after HS/T. There were no significant differences seen across groups, however, for a number of the tested biomarkers. It is possible that other time points than the one used in this study may better capture the immunomodulatory effects of MSCs and MSC EVs in this model, as MSCs and MSC EVs have been shown to have numerous immune-regulating effects in a variety of inflammatory diseases.24,25 In addition, others have shown initial systemic immune activation after MSC infusion in mice followed by reduced immune reactivity, further suggesting additional time points would be beneficial to understanding MSC and MSC EV immunomodulation after HS/T.26
MSCs and MSC EVs contain numerous bioactive factors, including mRNAs, microRNAs, proteins, and lipids, a combination of which may underly the vasculoprotective properties seen in this study. For example, MSCs and MSC EVs contain the mRNA angiopoietin-1 (Ang-1), a ligand for the Tie2 receptor tyrosine kinase which forms an important endothelial signaling pathway that inhibits vascular permeability and leukocyte-endothelium interactions.21,27,28 MSC EVs also contain a number of other factors such as hepatocyte growth factor (HGF), tissue inhibitor of metalloproteinase 3 (TIMP3), and sphingosine 1-phosphate (S1P), which have all been shown to have important roles in maintaining or restoring endothelial barrier function.28–30 EVs from MSCs exposed to culture conditions mimicking an ischemic microenvironment have also been found to contain an abundance of proteins involved in pathways including vascular wall cell surface interactions, cadherin signaling, cytoskeletal signaling, and vasculogenesis.31
There are several important limitations to this study. First, the Caco-2 cell line used in vitro is derived from colon adenocarcinoma, which may not entirely reflect the response of the small intestine to oxidative stress in vivo. However, the Caco-2 cell line in culture resembles enterocytes lining the small intestine, is used extensively to study the intestinal epithelial barrier, and is considered the in vitro gold standard for the assessment of drug permeability and absorption.32 Second, human rather than mouse MSCs and MSC EVs were used in a mouse model, which raises the possibility of an interspecies effect. This was done to best evaluate the product that would be given to humans (i.e. human MSCs and MSC EVs). MSCs are also considered immune evasive and have been used in this manner in other studies.33 Third, because there were no changes in tight junction staining in the intestinal epithelial barrier in vivo, we were unable to determine the impact of these therapies on the gut barrier. This was an unexpected finding given the clear decrease in pulmonary epithelial tight junction staining after HS/T, and others have demonstrated loss of intercellular junction integrity in the gut in similar models.34–36 Finally, a number of the plasma inflammatory biomarkers were unexpectedly not different between sham animal and the shocked groups, though this may not be surprising given that the sham animals in this study do undergo bilateral femoral artery cannulation and ligation, and therefore some degree of hindlimb ischemia, as well as 2 hours of anesthetic time.
In conclusion, this study demonstrates that both MSCs and MSC EVs help to maintain vascular barrier integrity in vitro and in vivo in a mouse model of HS/T. MSC EVs may therefore be able to recapitulate many of the potential therapeutic benefits of MSCs in HS/T in a cell-free manner, overcoming some of the logistical barriers and disadvantages of live cell administration in patients. MSC-based therapies are currently being evaluated in a variety of clinical settings, with published trials demonstrating positive results and a favorable safety profile.37 However, understanding the potential role of these therapies in HS/T is only in its infancy.9,38 A number of questions remain to be addressed, including cell source, dosing, timing of delivery, and the duration of their effects. Further studies are also needed to validate MSC-based therapies in critically ill patients with systemic inflammation and coagulopathy and in patients who are receiving simultaneous therapies such as blood products and hemostatic agents.
Acknowledgements:
Not applicable.
Funding:
Funding for this work was provided by NIH grant R01GM111899.
Footnotes
Competing Interests: The authors declare that they have no competing interests.
Ethics Approval: Animal studies were performed with approval of the Institutional Animal Care and Use Committee (IACUC) at UCSF. The experiments were conducted in compliance with the ARRIVE guidelines for animal models and the National Institutes of Health (NIH) guidelines on the use of laboratory animals.
Consent for Publication: Not applicable.
Contributor Information
Mark Barry, University of California San Francisco.
Alpa Trivedi, University of California San Francisco.
Byron Miyazawa, University of California San Francisco.
Lindsay Vivona, University of California San Francisco.
David Shimmin, University of California San Francisco.
Praneeti Pathipati, University of California San Francisco.
Callie Keane, University of California San Francisco.
Joseph Cuschieri, University of California San Francisco.
Shibani Pati, University of California San Francisco.
Availability of Data and Materials:
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Jenkins DH, Rappold JF, Badloe JF, et al. Trauma hemostasis and oxygenation research position paper on remote damage control resuscitation: definitions, current practice, and knowledge gaps. Shock. May 2014;41 Suppl 1:3–12. doi: 10.1097/SHK.0000000000000140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holcomb JB, Pati S. Optimal trauma resuscitation with plasma as the primary resuscitative fluid: the surgeon’s perspective. Hematology Am Soc Hematol Educ Program. 2013;2013:656–9. doi: 10.1182/asheducation-2013.1.656 [DOI] [PubMed] [Google Scholar]
- 3.Barry M, Pati S. Targeting repair of the vascular endothelium and glycocalyx after traumatic injury with plasma and platelet resuscitation. Matrix Biol Plus. Jun 2022;14:100107. doi: 10.1016/j.mbplus.2022.100107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naumann DN, Hazeldine J, Davies DJ, et al. Endotheliopathy of Trauma is an on-Scene Phenomenon, and is Associated with Multiple Organ Dysfunction Syndrome: A Prospective Observational Study. Shock. 04 2018;49(4):420–428. doi: 10.1097/SHK.0000000000000999 [DOI] [PubMed] [Google Scholar]
- 5.Johansson PI, Henriksen HH, Stensballe J, et al. Traumatic Endotheliopathy: A Prospective Observational Study of 424 Severely Injured Patients. Ann Surg. 03 2017;265(3):597–603. doi: 10.1097/SLA.0000000000001751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Langan NR, Eckert M, Martin MJ. Changing patterns of in-hospital deaths following implementation of damage control resuscitation practices in US forward military treatment facilities. JAMA Surg. Sep 2014;149(9):904–12. doi: 10.1001/jamasurg.2014.940 [DOI] [PubMed] [Google Scholar]
- 7.Cannon JW, Khan MA, Raja AS, et al. Damage control resuscitation in patients with severe traumatic hemorrhage: A practice management guideline from the Eastern Association for the Surgery of Trauma. J Trauma Acute Care Surg. March 2017;82(3):605–617. doi: 10.1097/TA.0000000000001333 [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. Web-based Injury Statistics Query and Reporting System (WISQARS). Accessed May 16, 2022. www.cdc.gov/injury/wisqars [Google Scholar]
- 9.Pati S, Pilia M, Grimsley JM, et al. Cellular Therapies in Trauma and Critical Care Medicine: Forging New Frontiers. Shock. Dec 2015;44(6):505–23. doi: 10.1097/SHK.0000000000000482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matthay MA, Pati S, Lee JW. Concise Review: Mesenchymal Stem (Stromal) Cells: Biology and Preclinical Evidence for Therapeutic Potential for Organ Dysfunction Following Trauma or Sepsis. Stem Cells. February 2017;35(2):316–324. doi: 10.1002/stem.2551 [DOI] [PubMed] [Google Scholar]
- 11.Pati S, Gerber MH, Menge TD, et al. Bone marrow derived mesenchymal stem cells inhibit inflammation and preserve vascular endothelial integrity in the lungs after hemorrhagic shock. PLoS One. 2011;6(9):e25171. doi: 10.1371/journal.pone.0025171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barry M, Trivedi A, Pathipati P, et al. Mesenchymal stem cell extracellular vesicles mitigate vascular permeability and injury in the small intestine and lung in a mouse model of hemorrhagic shock and trauma. J Trauma Acute Care Surg. 03 January 2022;92(3):489–498. doi: 10.1097/TA.0000000000003487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aussel C, Baudry N, Grosbot M, et al. IL-1β primed mesenchymal stromal cells moderate hemorrhagic shock-induced organ injuries. Stem Cell Res Ther. 08 May 2021;12(1):438. doi: 10.1186/s13287-021-02505-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Zhang X, Zhang H, et al. Mesenchymal Stem Cells Derived Extracellular Vesicles Alleviate Traumatic Hemorrhagic Shock Induced Hepatic Injury. Front Immunol. 2021;12:811164. doi: 10.3389/fimmu.2021.811164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gore AV, Bible LE, Livingston DH, Mohr AM, Sifri ZC. Mesenchymal stem cells reverse trauma and hemorrhagic shock-induced bone marrow dysfunction. J Surg Res. Dec 2015;199(2):615–21. doi: 10.1016/j.jss.2015.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eggenhofer E, Luk F, Dahlke MH, Hoogduijn MJ. The life and fate of mesenchymal stem cells. Front Immunol. 2014;5:148. doi: 10.3389/fimmu.2014.00148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rani S, Ryan AE, Griffin MD, Ritter T. Mesenchymal Stem Cell-derived Extracellular Vesicles: Toward Cell-free Therapeutic Applications. Mol Ther. May 2015;23(5):812–823. doi: 10.1038/mt.2015.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raposo G, Stahl PD. Extracellular vesicles: a new communication paradigm? Nat Rev Mol Cell Biol. September 2019;20(9):509–510. doi: 10.1038/s41580-019-0158-7 [DOI] [PubMed] [Google Scholar]
- 19.Baek G, Choi H, Kim Y, Lee HC, Choi C. Mesenchymal Stem Cell-Derived Extracellular Vesicles as Therapeutics and as a Drug Delivery Platform. Stem Cells Transl Med. 09 2019;8(9):880–886. doi: 10.1002/sctm.18-0226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barry M, Trivedi A, Miyazawa BY, et al. Cryoprecipitate Attenuates the Endotheliopathy of Trauma in Mice Subjected to Hemorrhagic Shock and Trauma. J Trauma Acute Care Surg. Mar 2021;doi: 10.1097/TA.0000000000003164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Potter DR, Miyazawa BY, Gibb SL, et al. Mesenchymal stem cell-derived extracellular vesicles attenuate pulmonary vascular permeability and lung injury induced by hemorrhagic shock and trauma. J Trauma Acute Care Surg. February 2018;84(2):245–256. doi: 10.1097/TA.0000000000001744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar H, Jo MJ, Choi H, et al. Matrix Metalloproteinase-8 Inhibition Prevents Disruption of Blood-Spinal Cord Barrier and Attenuates Inflammation in Rat Model of Spinal Cord Injury. Mol Neurobiol. Mar 2018;55(3):2577–2590. doi: 10.1007/s12035-017-0509-3 [DOI] [PubMed] [Google Scholar]
- 23.Bates DO. Vascular endothelial growth factors and vascular permeability. Cardiovasc Res. Jul 15 2010;87(2):262–71. doi: 10.1093/cvr/cvq105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kou M, Huang L, Yang J, et al. Mesenchymal stem cell-derived extracellular vesicles for immunomodulation and regeneration: a next generation therapeutic tool? Cell Death Dis. Jul 04 2022;13(7):580. doi: 10.1038/s41419-022-05034-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao F, Chiu SM, Motan DA, et al. Mesenchymal stem cells and immunomodulation: current status and future prospects. Cell Death Dis. Jan 21 2016;7(1):e2062. doi: 10.1038/cddis.2015.327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoogduijn MJ, Roemeling-van Rhijn M, Engela AU, et al. Mesenchymal stem cells induce an inflammatory response after intravenous infusion. Stem Cells Dev. Nov 01 2013;22(21):2825–35. doi: 10.1089/scd.2013.0193 [DOI] [PubMed] [Google Scholar]
- 27.Saharinen P, Eklund L, Alitalo K. Therapeutic targeting of the angiopoietin-TIE pathway. Nat Rev Drug Discov. Sep 2017;16(9):635–661. doi: 10.1038/nrd.2016.278 [DOI] [PubMed] [Google Scholar]
- 28.Hu S, Park J, Liu A, et al. Mesenchymal Stem Cell Microvesicles Restore Protein Permeability Across Primary Cultures of Injured Human Lung Microvascular Endothelial Cells. Stem Cells Transl Med. August 2018;7(8):615–624. doi: 10.1002/sctm.17-0278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang H, Zheng R, Chen Q, Shao J, Yu J, Hu S. Mesenchymal stem cells microvesicles stabilize endothelial barrier function partly mediated by hepatocyte growth factor (HGF). Stem Cell Res Ther. September 29 2017;8(1):211. doi: 10.1186/s13287-017-0662-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Menge T, Zhao Y, Zhao J, et al. Mesenchymal stem cells regulate blood-brain barrier integrity through TIMP3 release after traumatic brain injury. Sci Transl Med. Nov 2012;4(161):161ra150. doi: 10.1126/scitranslmed.3004660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anderson JD, Johansson HJ, Graham CS, et al. Comprehensive Proteomic Analysis of Mesenchymal Stem Cell Exosomes Reveals Modulation of Angiogenesis via Nuclear Factor-KappaB Signaling. Stem Cells. Mar 2016;34(3):601–13. doi: 10.1002/stem.2298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sambuy Y, De Angelis I, Ranaldi G, Scarino ML, Stammati A, Zucco F. The Caco-2 cell line as a model of the intestinal barrier: influence of cell and culture-related factors on Caco-2 cell functional characteristics. Cell Biol Toxicol. Jan 2005;21(1):1–26. doi: 10.1007/s10565-005-0085-6 [DOI] [PubMed] [Google Scholar]
- 33.Ankrum JA, Ong JF, Karp JM. Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol. Mar 2014;32(3):252–60. doi: 10.1038/nbt.2816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schucht JE, Matheson PJ, Harbrecht BG, Bond L, Ashkettle GR, Smith JW. Plasma resuscitation with adjunctive peritoneal resuscitation reduces ischemia-induced intestinal barrier breakdown following hemorrhagic shock. J Trauma Acute Care Surg. 01 January 2021;90(1):27–34. doi: 10.1097/TA.0000000000002916 [DOI] [PubMed] [Google Scholar]
- 35.Thuijls G, de Haan JJ, Derikx JP, et al. Intestinal cytoskeleton degradation precedes tight junction loss following hemorrhagic shock. Shock. Feb 2009;31(2):164–9. doi: 10.1097/SHK.0b013e31817fc310 [DOI] [PubMed] [Google Scholar]
- 36.Yang R, Han X, Uchiyama T, et al. IL-6 is essential for development of gut barrier dysfunction after hemorrhagic shock and resuscitation in mice. Am J Physiol Gastrointest Liver Physiol. Sep 2003;285(3):G621–9. doi: 10.1152/ajpgi.00177.2003 [DOI] [PubMed] [Google Scholar]
- 37.Rodríguez-Fuentes DE, Fernández-Garza LE, Samia-Meza JA, Barrera-Barrera SA, Caplan AI, Barrera-Saldaña HA. Mesenchymal Stem Cells Current Clinical Applications: A Systematic Review. Arch Med Res. January 2021;52(1):93–101. doi: 10.1016/j.arcmed.2020.08.006 [DOI] [PubMed] [Google Scholar]
- 38.Herzig MC, Cap AP. Challenges in translating mesenchymal stem cell therapies for trauma and critical care. Transfusion. Apr 2016;56(4):20S-5S. doi: 10.1111/trf.13566 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.