Abstract
Small leucine rich proteoglycans (SLRPs) are the largest family of proteoglycans, with 18 members that are subdivided into five classes. SLRPs are small in size and can be present in tissues as glycosylated and non-glycosylated proteins, and the most studied SLRPs include decorin, biglycan, lumican, keratocan and fibromodulin. SLRPs specifically bind to collagen fibrils, regulating collagen fibrillogenesis and the biomechanical properties of tissues, and are expressed at particularly high levels in fibrous tissues, such as the cornea. However, SLRPs are also very active components of the ECM, interacting with numerous growth factors, cytokines and cell surface receptors. Therefore, SLRPs regulate major cellular processes and have a central role in major fundamental biological processes, such as maintaining corneal homeostasis and transparency and regulating corneal wound healing. Over the years, mutations and/or altered expression of SLRPs have been associated with various corneal diseases, such as congenital stromal corneal dystrophy and cornea plana. Recently, there has been great interest in harnessing the various functions of SLRPs for therapeutic purposes. In this comprehensive review, we describe the structural features and the related functions of SLRPs, and how these affect the therapeutic potential of SLRPs, with special emphasis on the use of SLRPs for treating ocular surface pathologies.
1. Introduction
Small leucine rich proteoglycans (SLRPs) make up the largest class of proteoglycans (PGs) [1–8]. There are 18 members in the SLRPs family, which are encoded by 18 genes that can produce various glycosylated forms and spliced variants (Table 1). The most studied SLRPs include decorin, biglycan, lumican, keratocan and fibromodulin. As the names states, SLRPs contain various leucine rich repeat (LRR) domains that are flanked by clusters of cysteine residues which cause them to have their typical curved solenoid shape [8–14]. Compared to other extracellular proteoglycans, SLRPs are small in size, with the core proteins ranging from 36 to 77 kDa and can be present in tissues as glycosylated and non-glycosylated proteins [15–17]. SLRPs are ubiquitous, with key roles in development, homeostasis and pathology. They are expressed at particularly high levels in fibrous tissues, for example the cornea, tendons, ligaments, and fibrous septa and capsules that encapsulate major organs, such as meninges and pericardium [18–20]. To date, the first and main function that has been attributed to SLRPs is in organizing the ECM by regulating collagen fibrillogenesis, limiting collagen fibril lateral growth, maintaining correct intra and inter-fibrillar spacing and regulating collagen organization [21–23]. However, SLRPs are also very active components of the ECM, interacting with numerous growth factors, cytokines and cell surface receptors, and, therefore, can also regulate major cellular processes [2,6,7,15,24]. They have been shown to have a central role in cell adhesion, proliferation and differentiation, and, thus, there has been great interest in harnessing the various functions of SLRPs for therapeutic purposes in the fields of tissue regeneration and oncology [2,6,25,26]. SLRPs have been shown to interact with certain integrins and receptor tyrosine kinases [27–30], mediate signaling via various innate immune receptors [2,31], bind and modulate transforming growth factor beta (TGFβ) and bone morphogenetic protein (BMP), and modulate various signaling pathways [2,32–36]. Thus, many SLRPs, such as lumican, have been coined as matrikines [37]. Over the years, mutations and/or loss of SLRPs have been associated with various diseases, for example, congenital stromal corneal dystrophy, cornea plana and osteoarthritis. Decorin, biglycan, lumican, fibromodulin, keratocan, and osteoglycin (mimecan) have all been shown to be expressed and have important functions in the cornea. However, functions for opticin, osteoadherin, PRELP, epiphycan, chondroadherin, ECM2, ECMX, nyctalopin, podocan and podocan-like have yet to be explored in the cornea.
Table 1.
Organization of the family of SLRPs into Classes I–V.
| Class I | Class II | Class III | Class IV | Class V |
|---|---|---|---|---|
|
| ||||
| Decorin (DCN) | Lumican (LUM) Subgroup A | Epiphycan (EPN) | Chondroadherin (CHAD) | Podocan (PODN) |
| Biglycan (BGN) | Fibromodulin (FMOD) Subgroup A | Opticin (OPTC)/oculoglycan | Chondroadherin-like protein (CHADL) | Podocan-like (PODN1) |
| Asporin (aspn) | Keratocan (KERA) Subgroup B | Osteoglycin (OGN)/mimecan | Nyctalopin (NYX) | |
| Extracellular matrix 2 (ECM2) | Proline and arginine rich end leucine rich protein (PRELP)/prolargin Subgroup B | Tsukushi (TSK) | ||
| Extracellular matrix X (ECMX) | Osteoadherin (OMD)/osteomodulin Subgroup C | |||
1.1. Classification of SLRPs
Over the years, 18 members have been identified for the SLRP family, and these are grouped into five classes named class I–V, as shown in Table 1 and Fig. 1A [22,38–41]. The organization of the SLRPs into the different classes is based on evolutionary conservation, protein and gene homology, and chromosomal organization [41]. Class I includes biglycan, decorin, asporin, extracellular matrix 2 (ECM2), and extracellular matrix X (ECMX), class II includes lumican, keratocan, fibromodulin, proline and arginine rich end leucine rich protein (PRELP), and Osteoadherin (OMD), class III includes opticin, osteoglycin/mimecan, and epiphycan (EPN), class IV includes chondroadherin (CHAD), chondroadherin-like protein (CHADL), Nyctalopin (NYX), and tsukushi, and class V includes podocan (PODN) and podocan-like protein 1 (PODN1) [22,38]. SLRPs can contain one or more glycosaminoglycan side chains, including CS, DS and KS, or they can contain other N- or O-linked glycans. However, not all members exist as proteoglycans. Specifically, asporin, ECM2 and ECMX (class I), PRELP (class II), opticin (class III), and nyctalopin and tsukushi (class IV) are believed to not carry GAG side chains. Classes I-III are considered canonical genes, while classes IV and V are considered non-canonical. All SLRPs have homologous structures and share several physiological functions. The LRR region of the SLRPs are flanked N- and C-terminal caps, formed by disulfide-bonds, and these caps are substantially distinct among the different classes and absent in the classes IV and V [22]. Class II SLRPs can be substituted with KS or polylactosamine (unsulfated KS) side chains [42,43]. This class of SLRPs are highly expressed in the cornea, where they are primarily substituted with KS side chains.
Fig. 1.
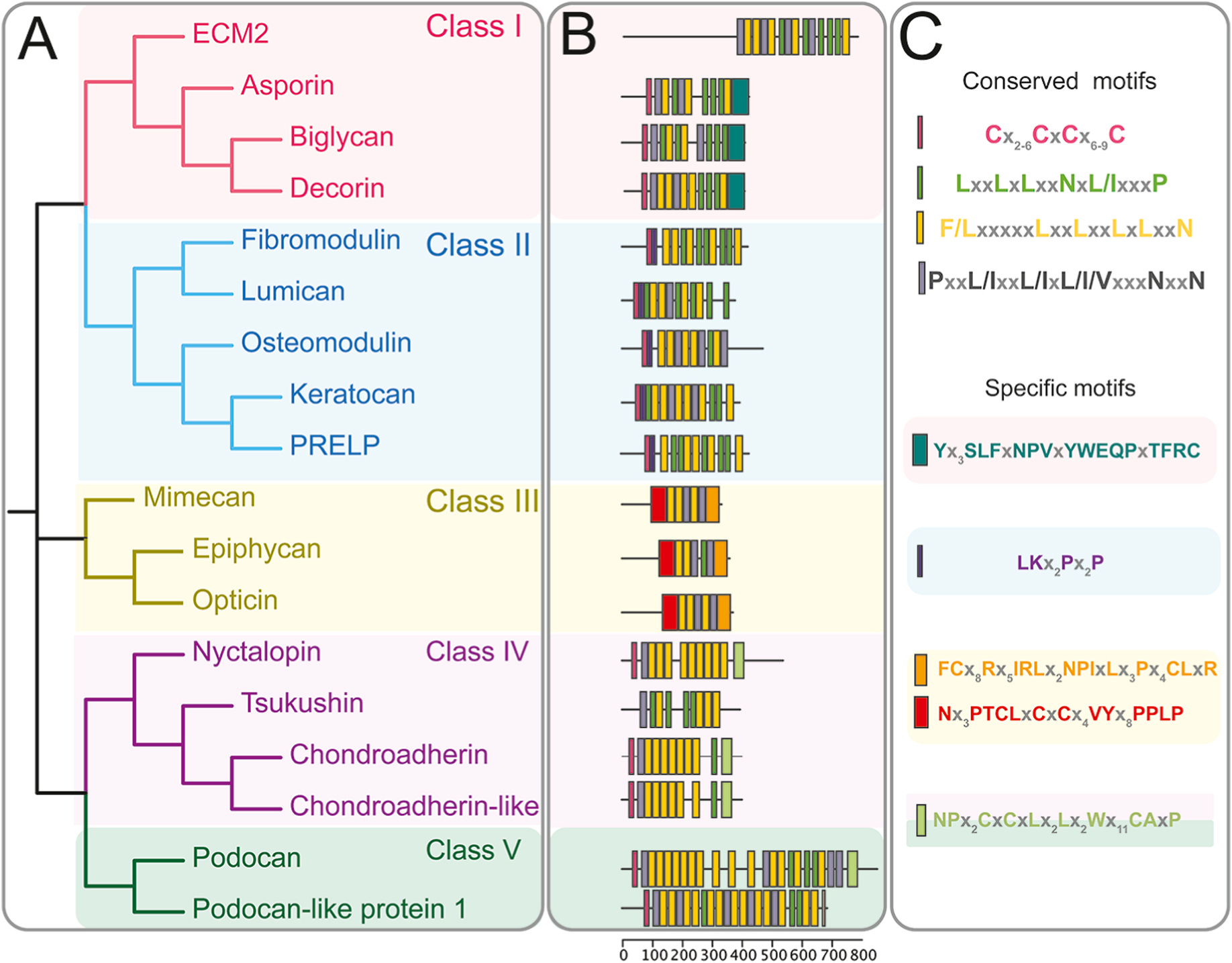
SLRPs motif conservation and distribution through phylogeny. (A) Dendrogram of five distinct families of SLRPs and related LRR proteins. (B) Distribution of conserved motifs and family specific motifs, as calculated by the multiple Em for motif Elicitation (MEME) suite [306]. (C) Sequence descriptor of graphical topology from B.
1.2. Structural features of SLRPs
SLRPs have a core protein that ranges from 33 to 77 kDa containing 12 to 22 leucine rich repeats arranged in tandem which are flanked by N- and C-terminal caps (Fig. 1). Each LRR repeat is 20–29 amino acids long, and individual repeats are composed by highly conserved segments (HCS) and variable segments (VS) which provide the functional binding diversity for SLRPs. The HCS are composed of LRRs with the following sequence patterns, LxxLxLxxNxL, LxxLxLxxNxxL or LxxLxLxxxL. The HCS are organized as a β-strand and loop followed by the VS that can form α-helices, 310-helices, polyproline helices, and β-turns. Together, the LRRs regions form the characteristic curved solenoid structure of SLRPs (Fig. 2). The curvature of the LRR structures results from differences in cross-sections between the b-strands on the concave side and the different helical structures on the convex side [44,45]. This curved architecture allows for the formation of protein-protein interaction modules where the concave surfaces of the LRR proteins contain ligand-binding sites. This hypothesis has been demonstrated in crystal structures of LRR proteins complexed with their ligands [14,46]. The curved shaped of SLRPs allows for the maximum number of contacts between the SLRPs and their ligands, with ligands with a radii smaller than the curvature of the concave face being enfolded by the SLRPs [47,48]. Over the years, numerous three-dimensional structures of complexes between LRR proteins or domains with their ligands have been determined [49–55].
Fig. 2.
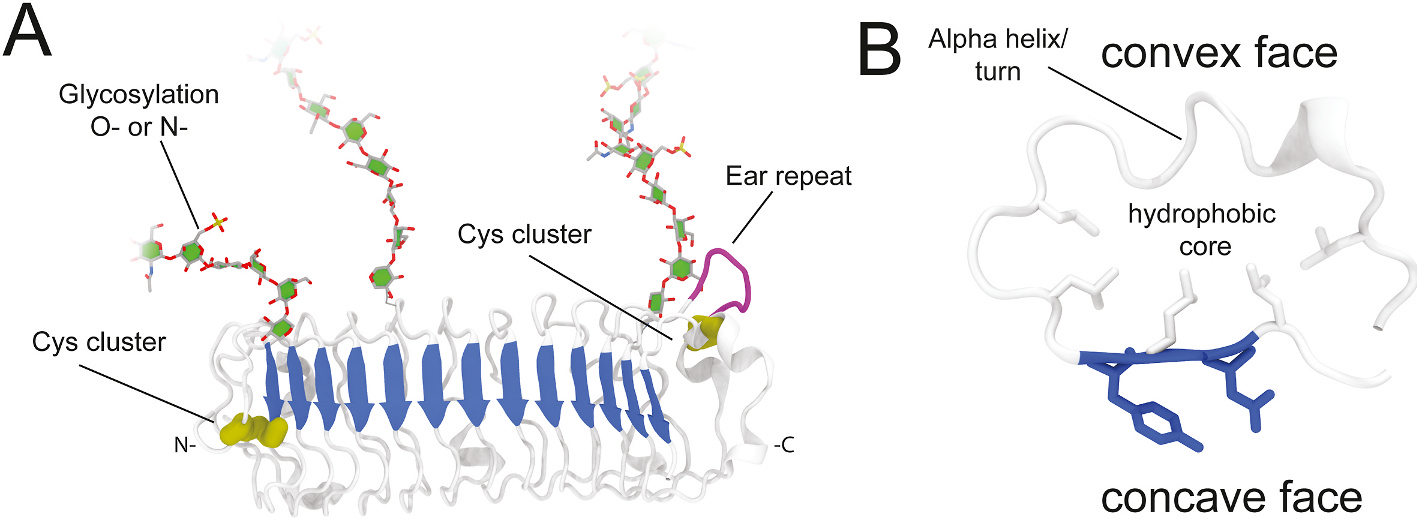
General structure of SLRPs. (A) SLRPs overall arched structure (white), with beta sheet on its concave side (blue) that participate in most of SLRP-ligand interactions. Some SLRPs show a N-terminal cysteine cluster (yellow) provides the structural stability for the protein to fold, and some also have a C-terminal cysteine cluster stabilize the folded protein. Additionally, some SLRPs have an “ear repeat” (purple) with unknown function. Other characteristics not displayed include proline, arginine and lysine amino acid clusters and tyrosine sulfation. Finally, chains can be N- or O-glycosylated. (B) Detail of a leucine rich repeat, convex shape defines curvature by the presence of alpha-helices (more curved) or turns (less curved). Fold is maintained by leucines at the hydrophobic core (white sticks) and binding is promoted by residues at the concave face (blue sticks).
Most SLRPs contain an N-terminal helical capping motif (LRRNT) and some contain a C-terminal cap (LRRCT), which flank the LRR domains [12,56]. For most SLRPs, 4 cysteine residues exist within the N-terminal region which form disulfide bonds to form the N-terminal cap, while 2 cysteine residues exist with the c-terminal region and form disulfide bonds forming the C-terminal cap [10,12]. It is primarily the positioning of these cysteine residues within the N-terminal region that dictates the organization of the different SLRPs into the five groups. The N- and C-terminal caps are essential for maintaining structure integrity of LRRs. The N-terminal cap folds at a faster rate than beta sheet domains during translation, controlling the overall folding of SLRPs. A laterally extended LRR repeat preceding the last LRR C-terminal repeat is atypical and contains disulfide bonds, which is unique to SLRPs [56]. This extension, referred as the “ear” domain, has a sequence that is distinct to other LRRCT motifs and was named LRRCE.
1.3. How structure dictates the functions of the different classes of SLRPs
In this section we will discuss the structural particularities of each class of SLRPs and correlate these unique structural characteristics with their functions. We will also highlight how molecules with such high sequence and structural homology evolved to bind different targets and have such distinct functions.
1.3.1. Class I SLRPs
Class I SLRPs have eight analogous exons with conserved intron/exon junctions (Fig. 1B). The number of LRR motifs can vary between different class I SLRPs, with decorin containing 12 LRR motifs, while biglycan contains 10 LRR motifs each (Fig. 1B). Decorin and biglycan are the most widely studied SLRPs, with the structure and function of each LRR of decorin characterized. Specifically, LRR V/VI assists in decorin binding to VEGFR2, LRR VII contains the collagen type I-binding sequence, LRR XII binds to CCN2/CTGF; and LRR XI is known as the “ear” repeat whose truncations or mutations may cause congenital stromal corneal dystrophy [14,15]. The CS/DS side chain is crucial for granting decorin and biglycan their regulatory effect on collagen fibrillogenesis [16], however this glycosylation has been shown to be dispensable for controlling intracellular signaling cascades by cell-surface receptors or signaling molecules. Studies have demonstrated that decorin may form dimers in physiological solutions, while it is biologically active as a monomer [36]. The non-specific self-association was shown to be caused by the removal of salts and water following dialysis and freeze-drying, and thus has been speculated not occur naturally in tissues [57]. Decorin proteoglycan and glycoprotein core preparations obtained in non-denaturing conditions demonstrate it is decorin monomers, and not dimers, that exhibit biological activity [36]. Both ECM2 and asporin genes are physically linked together on chromosome 9 [58]. ECM2 is characterized by 15 LRRs and 3 N-glycosylation sites, and binds to various extracellular proteins, including integrins [59], laminin [60], collagen types IV and V, proteoglycans, and various proteases [60,61]. However, the existence of specific motifs within the different ECM2 and asporin LRR domains that mediate these interactions have not been fully characterized.
1.3.2. Class II SLRPs
Human lumican, osteomodulin and fibromodulin have 11 LRR motifs, while keratocan has 10 LRR motifs and PRELP has 12 LRR motifs. Lumican has a negatively charged N-terminal domain (LRRNT), containing two potential sites for tyrosine sulfation [62], a central region containing the leucine-rich repeat domains, and a C-terminal sequence with 2 conserved cysteines and 2 leucine-rich repeats [62,63]. Lumican can exist in tissues as a proteoglycan, bound to keratan sulfate I (KS I) [64] or as a glycoprotein containing polylactosamine chains, short oligosaccharides, or poorly sulfated/unsulfated polylactosamine chains rather than KS [65]. As with other SLRPs, the highly variable N- and C-terminal regions of lumican dictate its physiological functions [66]. For example, the N-terminal region of lumican contains clusters of tyrosines that bind to CD14 [67] while the C-terminal region binds to extracellular surface receptors such as TGFBRI [68,69]. In contrast to decorin, fibromodulin does not dimerize in solution, and crystal packing shows a wide range of, presumably non-physiological, concave face interactions [70,71]. The LRRs 5–7 and 11 of fibromodulin have been found to be crucial for collagen binding [70,71] which are located on the C-terminus, leaving the N-terminal region of fibromodulin, which contains most of the surface-exposed aromatic residues, free to interact with other binding partners [72]. Osteomodulin contains 11 LRR motifs, with LRR9 and LRR10 suggested to be involved in weak electrostatic forces that promote binding to type I collagen [73], while LRRs 10 and 11 have been shown to be crucial for binding to BMP2 [74].
1.3.3. Class III-V SLRPs
Chondroadherin, a cartilage matrix protein that mediates the adhesion of isolated chondrocytes, is the only class III SLRP with a characterized structure. The chondroadherin core protein has 11 leucine-rich repeats (LRR) that are surrounded by cysteine-rich domains. Unlike the typical structure of canonical small leucine-rich proteoglycans (SLRPs), the crystal structure of human chondroadherin shows a well-organized C-terminal cap structure. This capping structure is similar to the LRRCT motif found in most LRR proteins, instead of the LLRCE motif which is unique to canonical SLRPs [75]. This C-terminal cap of chondroadherin includes the binding site for α2β1 integrin and is believed to play a functional role in cell adhesion. Although the structure of nyctalopin and tsukusi has still not been resolved, they present an increased number of residues between conserved cysteines, which is predicted to produce a longer β0-β1 loop.
When compared to other classes of SLRPs, the class V SLRPs have a higher number of LRR domains; human podocan has 20 LRR domains while and human podocan-like protein 1 has 17 LRR domains [76]. Podocan has a unique N-terminal cysteine-rich pattern and lacks the C-terminal cysteine residues that are characteristic of the LRRCT region. Podocan has a highly charged acidic C-terminal domain, which is believed to be of considerable importance with respect to its physiological role maintaining charge selection of the glomerular filtration barrier [77]. Further studies characterizing the structural features of all different SLRP classes will aid our understanding of their distinct roles within the extracellular matrix of tissues.
1.4. Post-translational modifications of SLRPs and their functions
In the post-genomic era, with the availability of genome sequences, focus has shifted towards understanding the dynamic structure and function of proteins. The specific functional form of a protein is dependent upon dynamic post-translational modifications (PTMs). PTMs refer to enzyme-mediated or covalent chemical modifications to proteins that can occur during or after their biosynthesis [78]. Currently more than 300 post-translational modifications are known, with most common among SLRPs being glycosylation, sulfation, and enzymatic cleavage [79,80].
1.4.1. Glycosylation
Glycosylation, the enzyme-catalyzed addition of sugar(s) to proteins, is one of the most common PTM, with over 50% of all human proteins being glycosylated [81]. The types of protein glycosylation are classified according to the amino acids that carry the carbohydrate moiety, for example, N-linked glycosylation where the oligosaccharide binds to a nitrogen of asparagine, C-linked glycosylation where the oligosaccharide binds to a carbon of tryptophan, O-linked glycosylation where the oligosaccharide binds to an oxygen of a serine or threonine, S-linked glycosylation where the oligosaccharide binds to a sulfur of cysteine [82]. SLRPs, like other proteoglycans, are glycoproteins that contain one or more glycosaminoglycans (GAGs). GAGs are long unbranched, negatively charged polysaccharides containing a repeating disaccharide unit having one of the two modified sugars, N-acetylgalactosamine (GalNAc) or N-acetylglucosamine (GlcNAc), and a uronic acid such as glucuronate (GlcA) or iduronate (IdoA) or a galactose residue in the case of keratan sulfate [43]. The GAGs are classified based on the composition of the disaccharide subunit (Table 2).
Table 2.
Classification of glycosaminoglycans.
| Type of GAG | Composition of disaccharide | Linkage | Present in SLRP [83] |
|---|---|---|---|
|
| |||
| Chondroitin sulfates (CS) | d-glucuronate (GlcA) and GalNAc-4- or 6-sulfate | β(1,3) | Decorin (1CS/DS) and biglycan (0–2 CS/DS) |
| Dermatan sulfates (DS) | l-iduronate (IdoA) or d-glucuronate (GlcA) plus GalNAc-4-sulfate; GlcA and IdoA sulfated | β(1,3) if GlcA, α(1,3) if IdoA | |
| Heparin and heparin sulfates (HS/HEP) | l-iduronate (IdoA: many with 2-sulfate) or d-glucuronate (GlcA: many with 2-sulfate) and N-sulfo-d-glucosamine-6-sulfate | α(1,4) if IdoA, β(1,4) if GlcA | - |
| Hyaluronates (HA) | d-glucuronate (GlcA) plus GlcNAc | β(1,3) | - |
| Keratan sulfates (KS) | galactose plus GlcNAc-6-sulfate | β(1,4) | Lumican (3–5 kS I), Keratocan (3–4 kS I), fibromodulin (2–5 kS I), and osteoglycin/mimecan (2–3 kS I) |
Importantly, the addition of a GAG chain onto the SLRP directly affects its physiological functions. For example, N-linked glycosylation, the most common form of glycosylation in SLRPs, and O-linked glycosylation, the second most common type of glycosylation in SLRPs, are important for the proper folding and stability of the protein, assembly of multimeric structures and modulating the binding of specific ligands. During biosynthesis in the Golgi apparatus, GAGs can be modified by sulfotransferase and epimerase enzymes allowing GAGs to have a rich structural variability and generate specific epitopes with varying sulfation and epimerization patterns [43]. The sulfotransferase enzymes utilize the sulfate donor 3′-phosphoadenosine-5′-phosphosulfate (PAPS) to catalyze the addition 2-, 6- and 3-O sulfates to HS/HEP and 2-, 4-, and 6-O sulfates to CS/DS, and 6-O sulfates to KS. Epimerases convert GlcA to IdoA, which is necessary for generating HEP and DS [43]. GAGs can be further modified in the ECM by extracellular endosulfatases (SULFs) [84,85]. The composition of the GAG moieties of SLRPs significantly varies in tissues depending on the stage of development, in aging and in pathological conditions [16].
Most GAGs are attached to the core protein via a tetrasaccharide linkage region which is composed of a glucuronic acid (GlcA) residue, two galactose (Gal) residues, and a xylose (Xyl) residue attached to the hydroxyl group of Ser residue in the protein, forming GAG(n)–GlcA–Gal–Gal–Xyl–Ser–protein [82]. The glycosylation process is initiated by the addition of the first xylose by xylosyltransferase using UDP-xylose as a donor. Thereafter, β4 galactosyl-, β3 galactosyl-, and β3 glucuronosyltransferase families of enzymes catalyze the transfer of two galactose residues. This intermediate then undergoes phosphorylation at the C-2 position of xylose and in the case of CS, sulfation of the galactose residues in a substoichiometric manner. Thereafter, the addition of β4-linked N-acetylgalactosamine to the linkage tetrasaccharide initiates CS assembly and the addition of α4-linked N-acetylglucosamine initiates HS assembly [86]. KS is not bound to proteins via this common tetrasaccharide, and instead is bound to proteins via unique tissue specific linkage regions and based on the nature of these linkage regions two types of keratan sulfate exist KS1 and II. KS I, which was originally detected in cornea, is N-glycan linked to proteins via asparagine and KS II (often referred to as the skeletal KS) is an O-glycan linked to serine or threonine [86]. The cornea contains KS I which has a long (~50) linear poly-N-acetyl lactosamine consisting of repeating units of the disaccharide [→ 3Galβ(1 → 4)GlcNAcβ(1 →], linked to an asparagine residue via a mannose containing branched oligosaccharide [87–89]. The process of glycosylation is non-template driven, highly dynamic, and complex process that plays crucial roles in development, physiology and pathology [82]. As far as glycosylation in SLRPs is concerned, it contributes to the structure & conformational stability [90], the secretory process [91], direct interaction and fibrillogenesis of collagen [92–95] and protection of collagen fibril from digestion, for example by cathepsin K [96]. The glycosylation of SLRPs plays vital role in the eye and its adnexal structures [88,89,97].
1.4.2. Tyrosine sulfation
Tyrosine sulfation involves the covalent attachment of a sulfate group to a tyrosine residues in trans-Golgi by the membrane-bound enzyme tyrosylprotein sulfotransferase [98]. It is one of the most common post-translational modifications of tyrosine residues that has been reported in secretory proteins, plasma membrane proteins, adhesion molecules, coagulation factors, plasma proteins, immune components, and G-protein-coupled receptors [99–101]. Protein tyrosine sulfation is necessary for the bioactivity and interaction of some proteins such as prohormone processing [102,103], chemokine receptor signaling [104–106], leucocyte adhesion [107–109]. In SLRPs, the sulfation of tyrosine residues has been observed at the N-terminal of three class II SLRPs i.e. fibromodulin (up to 9 sites), osteoadherin (up to 6 sites), and lumican (2 sites) using a combination of mass spectrometry studies [110]. In osteoadherin, sulfated tyrosine residues are also observed in the C-terminal. The tyrosine sulfation in fibromodulin and osteoadherin results in a highly negatively charged domain which shares functional similarity with heparin and thus binds with heparin-biding proteins such as MMP13, basic fibroblast growth factor-2, the NC4 domain of collagen IX, thrombospondin I and interleukin-10, oncostatin M, PRELP and chondroadherin [111]. The binding affinity depends on the position and number of sulfated tyrosine residues. These tyrosine sulfate mediated interactions are believed to be involved in the organization of the ECM and in the sequestration of growth factors, matrix metalloproteinases and cytokines [111]. The tyrosine-sulfated domain and the leucine-rich repeat domain of fibromodulin bind to collagen I at the N-terminus, and at 100 and 220 nm from the N-terminus thus regulating fibrillogenesis [112].
Although the exact biological role of sulfated tyrosine residues in SLRPs needs to be further explored, it is predicted that sulfated tyrosine residues have a role in regulating protein interactions, increasing protein stability and directing correct protein folding [110]. Opticin, a class III SLRP, has been shown to carry tyrosine sulfations in the retinal pigmented epithelium using immune-affinity purification, ectopic expression and barium hydroxide hydrolysis approaches [113]. Since opticin has anti-angiogenic properties [114] and can bind retinal growth hormone [115] and collagen [116], it is important to verify whether tyrosine sulfation alters its anti-angiogenic properties and/or its interaction with collagen and/or retinal growth hormone.
1.4.3. Enzymatic cleavage of SLRPs
Extracellular cleavage of SLRPs into potential pharmacologically active products adds another level of complexity to characterizing the biological functions of SLRPs. Both in vitro and in vivo studies have revealed that SLRPs are susceptible to cleavage by MMPs and other proteases, as reviewed by Zappia et al. (2020) [80]. MMP-2, MMP-3, MMP-7 and MMP-13 cleave decorin [117]; MT1-MMP and MMP-13 cleave lumican [118]; MMP-13 cleaves fibromodulin, biglycan and opticin [119–122]; MMP-1–2-3–7-8–9 cleave opticin with MMP-2 and MMP-7 being the most efficient [123]; and, MMP-9 & MMP-12 can cleave biglycan [124]. Besides MMPs, ADAMTS-4, ADAMTS-5, and granzyme B can cleave biglycan, decorin, and opticin [125,126] and serine protease HTRA1 can cleave chondroadherin [127]. Various groups have reported that cleavage of SLRPs in skeletal tissues leads to alterations in ECM homeostasis, biomechanical properties, and accumulation of growth factors [80]. The cleavage of SLRPs, for example, decorin by the proteases from inflammatory cells, releases cleavage SLRP fragments which can act as danger-associated molecular patterns (DAMPs) and interact with pattern recognition receptors including toll-like receptors 2 and 4 [128]. The effects of SLRP cleavage and SLRP products in the cornea remains to be investigated.
The catabolism of SLRPs is initiated by their internalization via receptor-mediated pathways and direction to the lysosome [80]. The putative receptors for endocytosis on the plasma and endosomal membranes include 51 kDa, 26 kDa [129] and 110 kDa receptors [130], IGFR [131] and the class A scavenger receptor [132]. For decorin, additional pathways i.e. the clathrin-dependent pathway and the Tfr/recycling pathway have been suggested for endosytosis [80]. The presence of GAGs [133], interaction with other ECM proteins [134], EGFR, the PI-3 kinase signaling [135], and the presence of lipid rafts [135] interfere with the endocytosis of SLRPs. The loss of function of lysosomal enzymes responsible for GAG degradation, leads to a family of lysosomal disorders named mucopolysaccharidoses (MPS) causing the accumulation of GAGs in various tissues, including the eye [136]. The ophthalmic complications of MPSs include ocular hypertension, glaucoma, corneal opacification, retinopathy, optic nerve abnormality and optic atrophy. In MPS, the GAGs accumulate both intra- and extracellularly in corneal epithelium, keratocytes, stroma, and endothelium, and disrupts the optically important arrangement of collagen fibrils [137].
Besides these post-translational modifications, an exploratory analysis using bioinformatics and biostatical tools by Zappia et al. (2020) has revealed that SLRPs shows bias towards codon usage which might fine-tune the translational response of the cells during stress conditions [80]. SLRP family members can be grouped in two clusters based on different codon usage patterns. Cluster 1 comprises of chondroadherin, biglycan, PRELP, fibromodulin, opticin, tsukushi, podocan, podocan like protein 1, and nyctalopin which all show a similarly biased codon usage pattern that is in contrast with cluster 2 members i.e. decorin, lumican, epiphycan, asporin, ECM2, osteoglycin, keratocan and osteomodulin. Authors also found that the codon usage biased patterns contrasts osteomodulin with the other members of the SLRP family for leucine and for the triplet glutamine, glutamate, and lysine amino acids residues [80]. However, experimental validation for these results is still warranted.
1.5. Functions of SLRPs in the ocular surface
The ocular surface forms an interface between the environment and interior eye, consisting of the cornea, corneoscleral limbus, conjunctiva, tear film & ocular glands. Since the ocular surface is in direct contact with the outside world, it is prone to injuries and infections [43]. SLRPs are essential for the formation of a transparent cornea that is necessary for vision. Additionally, SLRPs and their GAG side chains are important players in ocular surface protection, corneal wound healing and regeneration. Various SLRPs are integral during the corneal healing process, and exogenous SLRPs can be used to promote wound healing. The O-glycans facilitate the attachment of microbes preventing them from invading the ocular surface, while N-glycans play a vital role in intracellular transport of mucins, epitope exposure and barrier function [43]. Herein we describe the specific functions of the different SLRPs in the ocular surface, which have been summarized in Fig. 3.
Fig. 3.
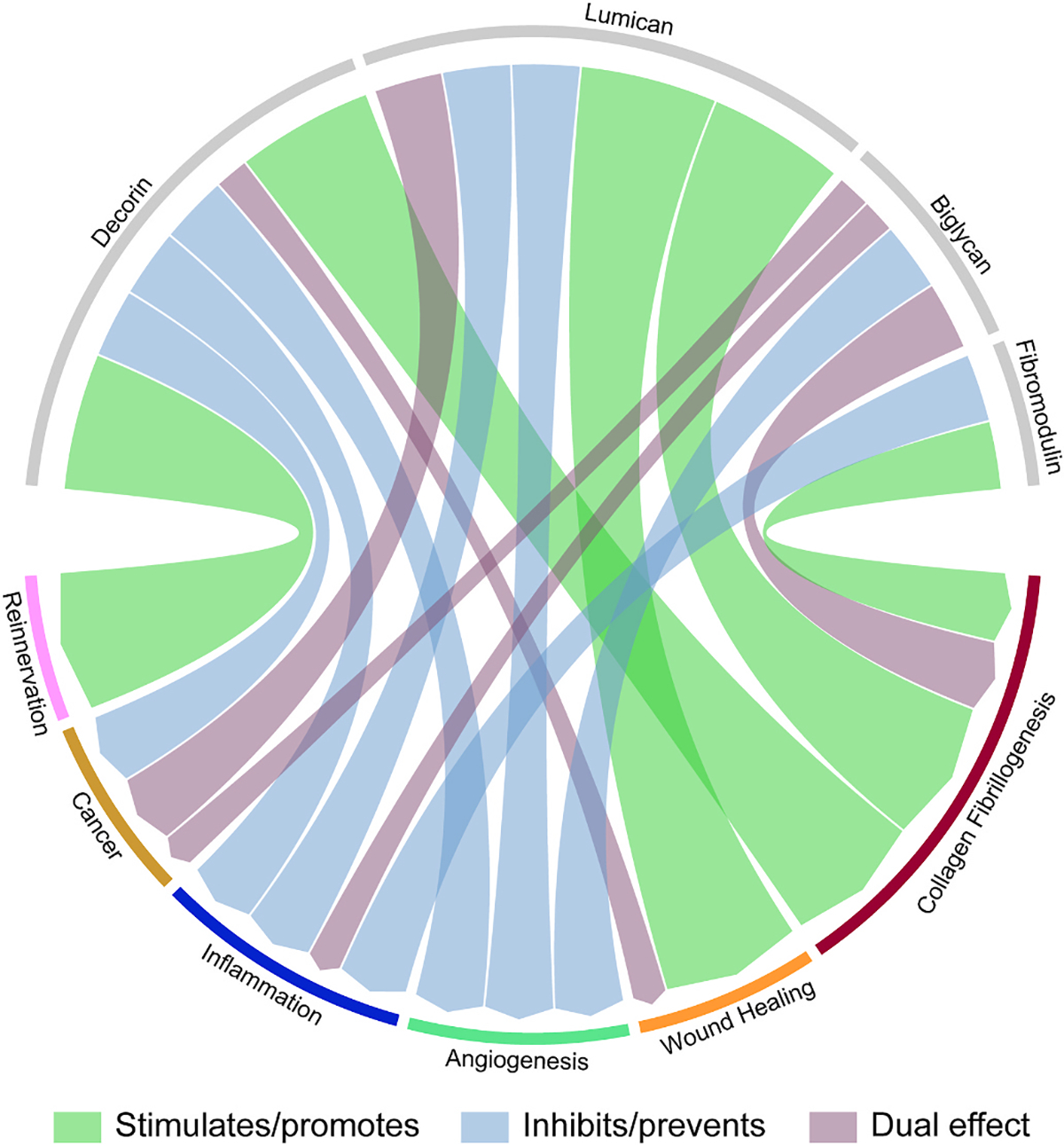
Circular plot of the various identified functions of decorin, biglycan, lumican, and fibromodulin. Green indicates stimulates or promotes; blue indicates inhibits or prevents and purple indicates a dual function, where, depending on the physiological condition it can sometimes stimulate/promote and sometimes inhibit/prevent.
1.5.1. Lumican
SLRPs are known to regulate collagen fibrillogenesis throughout the body. In the cornea, lumican regulates and maintains correct interfibrillar spacing of collagen fibers, which is vital for transparency, and Lum−/− mice develop bilateral corneal opacity, which is more severe in the posterior stroma [138]. Additionally, stromal thickness is reduced in Lum−/− mice. Fibromodulin also regulates corneal collagen fibrillogenesis, however mostly during corneal development [139], while in the adult mouse, fibromodulin is primarily expressed in the limbal region [140]. Lumican and fibromodulin are believed to have overlapping functions in the cornea, since the loss of both lumican and fibromodulin in Lum−/− ;Fmod−/− mice, leads to a more pronounced corneal phenotype than the Lum−/− and Fmod−/− mice alone. Lum−/− ;Fmod−/− mice also present scleral alterations and exhibit ocular features of high myopia, including scleral thinning and increased axial length [141]. Lumican also has been shown to regulate keratocan gene expression, and thus lumican null mice also present a decrease in keratocan expression [142]. In the cornea, lumican also plays a key role in regulating wound healing, with Lum−/− mice presenting delayed corneal wound closure [143]. During homeostasis lumican is expressed primarily in the corneal stroma, however, following corneal injuries, lumican expression is upregulated in the stromal and epithelial compartments, contributing towards promoting corneal epithelial cell migration and adhesion [143] and keratocyte proliferation and apoptosis [144].
Studies have demonstrated that it is specifically the core protein and not the KS side chains of lumican that promote corneal wound [145]. Specifically, the C-terminal region of lumican has been shown to bind to TGFβ receptor I (ALK5) to promote wound healing [68,69]. In an established cell line of human corneal epithelial cells, lumican has been shown to promote wound healing by stimulating cell migration via activating ERK1/2 and upregulating integrins α2 and β1 [68,146]. Lumican has also been shown to have a role in regulating the innate immune response, and this is also the case in the cornea. Lumican, biglycan and decorin have been shown mediate signaling via TLR-2 and 4, thereby influencing a major signaling axis that regulates a variety of host innate immune responses [67,147,148]. Lumican has been shown to work in concert with TLR-4 to increase the innate host immune response towards LPS, and lumican null mice present a decreased immune response to LPS-induced septic shock [149]. In the cornea, lumican null mice show reduced neutrophil and macrophage infiltration after a sterile LPS wound. Lumican null mice also show reduced neutrophil infiltration and poor bacterial clearance [144] during acute stages of bacterial keratitis, and, consequently, they display prolonged disease with increased inflammatory cell infiltration during latter stages [150]. Lumican has been shown to promote neutrophil chemotaxis towards LPS and CXCL1 via interactions with the αM (CD11b), β2 (CD18) and β1 (CD29) integrin subunits [149]. Lumican and keratocan have also been shown to directly bind to CXCl1 [142]. Decorin and biglycan likely have a similar role to lumican in regulating TLR signaling in the cornea, since, like lumican null mice, biglycan null mice also present a decreased responsive to LPS [131]and decorin enhances macrophage response to LPS in a TLR-2 and −4 dependent manner [31]. Both lumican and decorin expression have been found to be negatively correlated with corneal vascularization. Lumican and decorin expression are downregulated in mouse and human during corneas neovascularization, and their expression returns to normal levels as vessels regress [151]. Additionally, lumican has been shown to inhibit angiogenesis by interfering with α2β1 integrin activity [152].
Additionally, the KS side chain attached to lumican, and other class II SLRPs, also exerts important physiological functions. The negatively charged KS side chains of SLRPs regulate corneal hydration and transparency, development, and wound healing [64,88]. For normal collagen matrix biosynthesis and organization in the cornea, sulfation of KS by GlcNAc-6-O-sulfotransferase (GlcNAc6ST) is required [153,154], and reduced activity of GlcNAc6ST results in low-sulfated KS synthesis and accumulation in macular corneal dystrophy [154]. Transition from non-sulfated glycoprotein form to sulfated proteoglycan form of KS is shown to be concomitant with corneal transparency in chick [155,156] and mice [157] development. Studies have clearly demonstrated that the expression levels of SLRPs and their glycosylation dynamically change throughout development, aging and pathology [157,158]. Biglycan, decorin, lumican, fibromodulin and osteomodulin show an increase in non-glycanated forms with age [65,159,160], however, these changes in expression have never been explored in the ocular surface.
1.5.2. Keratocan
Keratocan is another major SLRP that is expressed in the cornea. In the cornea, keratocan expression is believed to be restricted to keratocytes, and thus it can be used as a putative keratocyte marker [160]. The development of Keratocan-Cre (Kera-Cre) and doxycycline inducible Keratocan-rtTA (KeraRT) transgenic driver mice are highly specific mouse models that can be used to ablate genes in stromal keratocytes [37,161]. Keratocan-null mice have clear corneas with thinner stroma that present abnormal collagen fibril spacing [142]. In humans, mutations in the keratocan gene have been linked to cases of cornea plana [162]. During the process of cornea lesion repair, the provisional matrix formed contains less keratocan and increased decorin and chondroitin sulfate levels [163]. The growth factors TGF-β1 and FGF-2 are responsible for many of the phenotypic changes in the activated keratocytes after cornea injury, including the downregulation or the loss of the expression of KSPGs [163]. Using a rabbit alkali burn model, lumican, keratocan and decorin were shown to have increased expression 1 and 3 months after injury, which are then down regulated at 6 months after injury [164]. Like lumican, keratocan has also been shown to regulate the innate immune response. Combined lumican and keratocan null mice are hyporesponsive to LPS, and present reduced stromal neutrophil infiltration [142].
1.5.3. Fibromodulin
Fibromodulin, another major KS-SLRP, is expressed in the cornea at significantly lower levels when compared to lumican and keratocan. Lumican and fibromodulin present 50% sequence homology and share the same binding site on collagen I [71]. Both lumican and fibromodulin have overlapping functions, and fibromodulin null mice have an attenuated phenotype through the compensatory activity of lumican. Fibromodulin is expressed in the peripheral cornea and posterior stroma during development, whereas it is expressed at low levels in the mature cornea, primarily in the limbal region [140,141]. Fibromodulin is expressed at high levels in the sclera and is believed to have a key role in regulating eye growth, and thus changes in fibromodulin expression could be involved in myopia progression [140].
Fibromodulin has been shown to interact with a variety of binding partners, including growth factors, enzymes, and structural proteins [165]. These interactions can occur through direct binding or through the modulation of signaling pathways [166]. Fibromodulin has been shown to bind TGF-β and modulate its activity [167]. Fibromodulin has been shown to bind and inhibit MMPs, which can have important consequences during tissue repair and remodeling [168]. The binding of fibromodulin to type I collagen fibrils affects the mechanical properties of tissues, such as tendons and ligaments, and may be important in the development and maintenance of these tissues [140]. Additionally, fibromodulin interacts with other ECM proteins, such as proteoglycans and glycoproteins, via the core protein and GAG chains, which have been shown to regulate cell behavior and tissue organization [140].
1.5.4. Mimecan
In the cornea, mimecan (osteoglycin) is mostly expressed in the epithelium and epithelial basement membrane [138]. Mimecan null mice present normal corneas with minimal changes in collagen fibril diameter and interfibrillar spacing [169].
1.5.5. Decorin
Decorin is a versatile protein that plays a critical role in regulating the activity of a wide range of targets. Decorin is highly expressed in the mature cornea, and its expression is homogenous throughout the cornea [170]. Like other SLRPs in the cornea, decorin has a major role in regulating the organization and structure of collagen fibrils throughout the stroma, which is severely disrupted in decorin null mice [171]. Studies have indicated that decorin and biglycan have overlapping functions in the cornea, with decorin null mice upregulating biglycan in the cornea leading to an attenuated phenotype [171]. The importance of decorin in the cornea was further demonstrated when mutations in the decorin gene were linked to human autosomal-dominant congenital corneal stromal dystrophy [172] which was partially recapitulated in a mouse model [173].
Decorin has been shown to bind and interact with integrins, growth factors, cytokines, adhesion molecules, and other glycoproteins, via which decorin regulates cell adhesion, migration, and signal transduction, maintains structural integrity of tissues [174]. It has been well established that decorin and various other SLRPs, including biglycan, fibromodulin, asporin, and tsukushi, bind and neutralize TGFβ [175–179]. SLRPs can regulate TGFβ signaling by sequestering TGFβ isoforms in the ECM, thus regulating their bioavailability [175,176, 178]. Given that TGFβ is a major driver of corneal fibrosis and myofibroblast generation, SLRPs have an important role in regulating corneal wound healing via regulation of TGFβ signaling and limiting corneal scarring. Decorin, and other SLRPs that bind TGFβ isoforms such as biglycan, and fibromodulin, are upregulated in the corneal stroma after injury [175,180,181]. Decorin and fibromodulin primarily accumulate in the anterior stroma, while biglycan is expressed in the epithelium and throughout the stroma [182]. Decorin gene therapy using an adeno-associated viral vector successfully attenuates myofibroblast transformation and fibrosis following epithelial-stromal injury [183, 184]. Overexpression of decorin has been shown to inhibit neovascularization of rabbit corneas, by down-regulating VEGF and angioprotein [185].
1.5.6. Biglycan
Biglycan has been shown to bind to various growth factors, cytokines, and adhesion molecules, via which biglycan can modulate various cell signaling pathways and cell functions [7,186]. Biglycan is a well-established ligand of the integrin, cadherins, and selectin families [187], and can bind to growth factors such as fibroblast growth factor (FGF), transforming growth factor (TGF), and platelet-derived growth factor (PDGF), via which biglycan mediates cell-cell interactions and regulates cell proliferation, differentiation, and migration. Biglycan is highly expressed in the cornea during early developmental stages, while it is expressed at lower levels in the mature cornea evenly distributed throughout the stroma [171]. Biglycan is upregulated after corneal injury [188]. Although biglycan is known to regulate collagen fibrillogenesis in other tissues, it has been speculated that it does not have a significant contribution towards the organization and structure of collagen in the cornea since biglycan null mice present normal distribution and organization of collagen fibrils in the mature cornea [171]. Biglycan is homologous to decorin, and they share a similar binding site on collagen, and thus decorin likely compensates for the loss of biglycan in biglycan null mice [186,187]. In support of this, double decorin and biglycan null mice, show large irregular collagen fibrils throughout the corneal stroma, especially in posterior regions, which is more pronounced than what is observed in the individual null mice [171]. Further, decorin null mice present an increase in biglycan expression through a compensatory mechanism [171]. During development, biglycan regulates the formation of a TGF-α gradient during eyelid morphogenesis [189]. Mice overexpressing biglycan under the keratocan promoter present precocious eyelid opening and lack Meibomian gland formation caused by biglycan binding to TGFα and interrupting EGFR signaling [189]. These mice also present exposure keratitis and alterations in eyelid muscle formation [189]. Biglycan has also been shown to regulate the immune response by binding and regulating the activity of various cytokines.
1.5.7. Opticin, osteoadherin, PRELP, asporin, ECM2, and ECMX
Opticin was first identified in the eye, most notably in the ciliary body by non-pigmented ciliary body epithelial cells [190] and even in advanced proliferative retinal disease, the non-pigmented ciliary body epithelial cells continue to express and secrete opticin [191]. No reports have attributed any functions to opticin in the cornea.
Osteoadherin is highly expressed in mineralized tissues. In bones, it is primarily expressed in the unglycanated form in non-mineralized zones and in the KSPG form in the mineralized zones [192]. Osteoadherin is also involved in odontogenesis, localized in the predentin region where is generates a gradient regulating mineralization [120,193]. No studies have reported functions for osteoadherin in the cornea.
PRELP was originally identified in the cartilage, and is highly expressed in the territorial matrix, and it has also been shown to be expressed in other tissues, primarily in and around basement membranes [194,195]. Recently, the use of PRELP for treating macular degeneration in mice has been explored using AAV-mediated delivery of human PRELP to inhibit the activation of the complement and prevent choroid angiogenesis [196]. However, no studies have reported any functions for PRELP in the cornea.
Asporin has been shown to play a critical role in cell adhesion and migration, cell signaling, and tissue development and remodeling [197]. As other SLRPs, asporin binds to lectins, collagen, laminin, and fibronectin, growth factors, via which interactions it is able to regulate cell growth and differentiation, and modulate inflammation [198]. Asporin has also been shown to bind to several enzymes, including elastase, hyaluronidase, and proteases [199]. Asporin has a major function in mineralization, and, thus, is highly expressed in mineralized tissues and has an N-terminal region that binds calcium and regulates hydroxyapatite formation. Functions for Asporin have never been explored in the cornea.
Loss of nyctalopin has been associated with congenital stationary blindness, characterized by reduced nocturnal vision [200,201]. Congenital stationary blindness has also been associated with myopia and reduced visual acuity. No functions have been attributed to nyctalopin in the cornea. Additionally, functions for epiphycan, chondroadherin, ECM2, ECMX, podocan and podocan-like in the cornea have never been investigated. ECM2 is mainly involved in the assembly and maintenance of the ECM [202].
1.6. Therapeutic and pharmacological applications of SLRPs
Due to their important roles in regulating the matrix remodeling, maintaining homeostasis and as effectors of several signaling pathways, over the past decade the biomedical interest in SLRPs has grown exponentially. Herein we will discuss the most recent advances in the therapeutic use of SLRPs.
Member of the class I SLRPs are distributed in the ECM of most tissues and play critical roles in tumor proliferation, migration, invasion, and angiogenesis. Decorin was coined as the “guardian of the matrix” [203] and has potential therapeutic use due to its antagonistic effects on TGF-β1 in numerous maladies including hydrocephalus [204], peritoneal fibrosis [205], diabetic nephropathy [206], abdominal aortic aneurysm [207], colorectal cancer [208], bleomycin induced lung fibrosis [209], glaucoma [210] and deep burns and skin scarring [211]. A decorin drug produced by Catalent pharma under the trade name galacorin®, has been tested for the treatment of macular degeneration, diabetic retinopathy, and diabetic macular edema [212]. Currently, galacorin is listed in numerous patent applications, being used alone or in combination with other proteins, such as anti-VEGF, and antibodies or fragments that bind to checkpoint inhibitor proteins such as CTLA-4 (Cytotoxic T-lymphocyte antigen 4), PD-1 (Programmed cell death protein 1), PD-L1 (Programmed death-ligand 1), PD-L2 (Programmed death-ligand 2), among others [213]. Decorin is a pan-receptor tyrosine kinase inhibitor, exerting oncosuppressive effects by regulating multiple receptors and diverse signaling pathways [214]. Several preclinical studies in cancers, especially of epithelial origin (which have not yielded any clinical trials as of the publication of this article), and the investigation of the molecular mechanisms underlying decorin’s anti-tumorigenic properties may lead to the development of novel decorin-based therapeutics with potential to revolutionize the treatment paradigm for various malignancies. However, it is important to note that the translation of these preclinical findings to clinical applications necessitates a thorough evaluation of decorin’s safety, efficacy, and pharmacokinetic profiles through well-designed clinical trials [215–218].
The therapeutic applications of decorin in preclinical stage include.
Anti-fibrotic properties: Decorin can attenuate the progression of fibrosis in organs such as the liver, kidney, and lung, by inhibiting the transforming growth factor-beta (TGF-β) signaling pathway, which plays a central role in the deposition of extracellular matrix components.
Anti-inflammatory effects: Decorin can suppress inflammation by modulating the activity of pro-inflammatory cytokines and signaling pathways. It may have potential applications in managing chronic inflammatory diseases, such as rheumatoid arthritis and inflammatory bowel disease.
Anti-tumorigenic activity: Decorin has demonstrated the ability to inhibit tumor growth and metastasis by targeting key signaling pathways involved in cancer cell proliferation, angiogenesis, and migration, such as the TGF-β and vascular endothelial growth factor (VEGF) pathways. Furthermore, it may enhance the sensitivity of cancer cells to chemotherapy and radiotherapy, suggesting its potential as an adjuvant therapy.
Wound healing and tissue repair: Decorin has been shown to promote wound healing and tissue repair by regulating cell migration, adhesion, and extracellular matrix remodeling. This property may be relevant for treating various cornea and skin conditions and injuries, as well as facilitating the healing of surgical wounds.
Neuroprotection: Some studies suggest that decorin may have neuroprotective effects by modulating neuroinflammation and promoting neurite outgrowth. This could lead to potential applications in the treatment of neurodegenerative diseases, such as Alzheimer’s and Parkinson’s disease.
Given that decorin has a role in various fundamental processes, such as, inhibiting angiogenesis [219], suppressing inflammation [220], promoting wound healing [221], preventing fibrosis [222], and inhibiting cancer progression [223], there has been great interest generating decorin based therapies for treating various diseases. As such, various therapeutic applications have been explored for decorin, and it is the SLRP with the highest number of described biological and medical uses. Decorin has been explored in a wide range of medical applications, from scarless wound healing to increasing muscle mass in muscular dystrophies [224,225].
Curiously, eye research has been at the forefront of developing decorin based therapies. The Mohan group and others have explored the use of decorin for promoting corneal wound healing and preventing scarring [1,184]. Decorin suppresses TGFβ signaling, and, as such, has the ability to delay collagen fibrillogenesis and prevent ECM deposition, thereby preventing corneal scarring [226]. In fact, Hill and colleagues designed gellan based fluid gels for sustained delivery of human recombinant decorin through eye drops to promote corneal regeneration [227]. Decorin based hydrogels have also been used to promote scarless regeneration in mice and rats [25,228]. Galacorin has been shown to effectively enhance stromal organization and increase biomechanical strength of the cornea, and has been proposed as a means to prolong myopia correction following orthokeratology lens (Ortho-K) wear [229, 230]. The Scar Free Foundation is to perform the first-in-human clinical trial of a new wound dressing loaded with decorin [231] in collaboration with Catalent, using a developed dressing containing Galacorin [212]. Galacorin is also being explored for the treatment of macular degeneration, diabetic retinopathy, and diabetic macular edema [212]. In addition to studies using decorin to promote scarless wound healing, many studies have explored the use of decorin for inhibiting corneal neovascularization [26,232,233]. The use of decorin for inhibiting neovascularization and/or fibrosis via gene therapy has been validated in the cornea [185,233], brain [234,235], kidney [236], and skin [237] with the mechanism likely being via decorin inhibiting TGFβ and VEGFR2. Because of the relatively small molecular weight of decorin, there is great potential for using decorin based therapies as an alternative to larger anti-VEGF mAb treatment, such as Avastin. The Mohan group has made great advances characterizing the biological role of decorin in the cornea [1,26,170,238] and has pioneered the therapeutic use of decorin for treating corneal neovascularization [185], inhibiting corneal scarring [184], and suppressing fibroblast transdifferentiation into myofibroblasts [183,239,240] His group has recently confirmed the safety of AAV5-Decorin targeted therapy for retarding corneal neovascularization in rabbits [233]. Decorin has also been used in a pilot study of experimental glaucoma filtration surgery using the rabbit model [221]. Postoperative results showed that rabbits treated with decorin had significantly less ECM deposition 14 days after surgery and less conjunctivital scarification.
Biglycan, like many other class-I and II SLRPs, has been reported to have therapeutic potential in multiple diseases. Specifically, biglycan has been used to treat muscular dystrophies by stabilizing the dystrophin associated protein complex (DAPC) and inducing phosphorylation of muscle specific tyrosine kinase (MuSK) [241,242]. Biglycan can also induce aggregation of acetylcholine receptors (AChRs) in neuromuscular or neurological diseases [241,242]. Human recombinant non-glycanated biglycan, TVN-102, is currently being developed as a therapeutic candidate for the treatment of Duchenne Muscular Dystrophy and Becker Muscular Dystrophy by Tivorsan Pharmaceuticals, Inc., USA, and the U.S. Food and Drug Administration (FDA) granted orphan drug designation to TVN-102 in 2016 [241,242]. TVN-102 upregulates utrophin, neuronal nitric oxide synthase (nNOS) and other dystrophin-associated proteins in the muscle membrane, and controls the activity and localization of MuSK at the nerve-muscle synapse [243].
Additionally, a formulation of one or more non-glycanated class I SLRPs have been shown to have therapeutic potential for treating neuromuscular disorders [244]. Biglycan acts as a danger signal in cancer, as it can interact with the toll-like receptor 2 and 4 on immune cells, causing a proinflammatory response by inducing the NLRP3 inflammasome, activation of caspase-1 and the release of various cytokines and chemokines [245]. Altered biglycan expression has been reported in different diseases including multiple types of solid cancers [246], and the potential of biglycan as a novel therapeutic target or agent for the treatment of inflammatory diseases, skeletal muscular dystrophies, muscle fiber regeneration has been investigated [247]. In breast cancer, biglycan activates the NF-κB signaling pathway, which is a transcriptional activator of PD-L1 [248], and the deletion of stromal biglycan enhances the efficacy of chemotherapy treatment [249]. It has been identified as a prognostic marker and potential therapeutic target for triple-negative breast cancer [250]. Biglycan knockout (Bgn KO) mice when injected with E0771 breast cancer cells, showed inhibited metastasis to the lung, impaired tumor angiogenesis, normalized tumor vasculature, suppressed fibrosis and increased CD8+ T cell infiltration compared to the wild type. Furthermore, delivery and efficacy of chemotherapy drugs were improved in vivo in Bgn KO mice [249]. In osteosarcoma cells, the binding of biglycan to lipoprotein receptor-related protein 6 activates the Wnt pathway and β-catenin nuclear translocation by disrupting β-catenin degradation complex, inhibiting Rapamycin-induced autophagy [251] Therefore, biglycan could be used as an adjuvant to improve the effectiveness of existing treatments for bone cancer by targeting vital signaling, including autophagy-related signaling, that contribute to chemotherapy resistant phenotypes [252,253]. Additionally, bigylcan has been explored as a means to treat atherosclerosis. In fact, an active patent application exists in Europe covering preventive and/or therapeutic effects of increased biglycan activity in atherosclerotic diseases [254]. This patent application covers the use of biglycan or enhancers of biglycan activity in pharmaceutical compositions with anti-atherosclerotic and anti-ischemic effects. Biglycan is believed to prevent the formation of the atherosclerotic plaque, such as coronary sclerosis, carotid artery sclerosis and peripheral artery sclerosis [254]. Besides having a great potential as biomarker in various diseases, biglycan has been shown to induce inflammation, regulate autophagy, mediate angiogenesis, promote cancer proliferation, invasion & metastasis and hence holds promising therapeutic implications for cancers [255–259].
Other patents also exist for the use of biglycan in various medical applications, including the use of biglycan as a diagnostic tool for assessment of heart failure [260], for prevention and treatment of obesity [261], diabetes [262] and associated pathologies [263]. To the best of our knowledge, no research exists and there are no patent applications covering the use of biglycan in medical applications that are related to the ocular surface. Curiously, biglycan, like other closely related SLRPs, i.e. decorin and fibromodulin (all having a leucine-rich repeat of about 24 amino acids), bind and inhibit TGFβ1 and morphology restoring factor (MRF), which in turn prevents/reduces scarring after wounding [264]. Thus, biglycan has the potential to be used in corneal wound healing to prevent/limit scarring, which results in loss of corneal transparency.
The main function attributed to lumican is regulating collagen fibrillogenesis, however it is also involved in various other tissue functions. Lumican regulates the extravasation of inflammatory cells and angiogenesis, and several studies have discussed targeting lumican to develop anti-cancer therapies to treat various forms of cancer, such as, pancreatic cancer [265], melanoma [266] and lung adenocarcinoma [5, 267]. In many cancer cell lines, such as pancreatic and breast cancer, lumican has been shown to inhibit migration [268]. However, lumican has also been shown to enhance the adhesion and migration of cells in a TGFβ dependent manner [269,270]. Lumican has also been shown to regulate adhesion and migration via integrin α2β1-collagen type I in a cell-specific manner [271]. Currently lumican holds its greatest potential as a prognostic marker in various pathologies [272,273] Lumican has been shown to have a protective role in sepsis, where injured epithelial cells secrete soluble lumican into the circulatory system that adheres to macrophages and is internalized via endocytosis, promoting TLR4 signaling, while suppressing TLR9 signaling [274]. To the best of our knowledge, this was the first report of a SLRP binding to DNA, opening new research avenues for designing immunosuppressive drugs. In the eye, studies have investigated the association between lumican and corneal dystrophies, such as keratoconus [199]. Dysregulated expression of lumican has been implicated in the abnormal ECM remodeling and biomechanical weakening of the cornea observed in keratoconus [275]. Additionally, lumican has been linked to the development and progression of myopia, a prevalent refractive error affecting visual acuity. Altered lumican expression in the sclera has been reported in both animal models of myopia and human subjects [276], suggesting that lumican may contribute to the biomechanical and structural changes observed in the sclera during myopia development.
The role of lumican in ocular wound healing and tissue repair has also been extensively explored. Lumican has been shown to modulate corneal epithelial cell migration, adhesion, and proliferation during the wound healing process [146,277,278], as well as influence the synthesis and deposition of ECM components, such as collagens and proteoglycans [279]. In corneal injury models, lumican-deficient mice exhibit delayed corneal re-epithelialization and impaired wound healing [143], emphasizing the importance of lumican in the maintenance and restoration of corneal integrity. Moreover, lumican has been implicated in ocular inflammation and angiogenesis. In the context of inflammatory corneal neovascularization, lumican has been reported to exert anti-angiogenic effects by inhibiting the actions of pro-angiogenic factors, such as vascular endothelial growth factor (VEGF) [280]. This suggests that lumican may serve as a potential therapeutic target for pathological neovascularization in ocular diseases, such as corneal graft rejection and neovascular age-related macular degeneration. Lumican also binds to ALK5 (Activin Receptor-Like Kinase 5), also known as TGFBR1, a crucial receptor in the TGF-β signaling pathway [68]. Dysregulation of TGF-β signaling has been implicated in the pathogenesis of multiple ocular diseases, such as corneal fibrosis, keratoconus, and posterior capsule opacification. Our group employed a rational design strategy to identify specific lumican-derived peptides capable of modulating the ALK5/TGFBRI pathway [69]. By utilizing in silico molecular docking to predict the binding of lumican peptides to the ALK5 receptor, we subsequently synthesized and tested the most promising peptide candidates in an in vivo model for cornea wound debridement. The results demonstrated that the selected peptides could indeed inhibit the kinase activity of ALK5 to promote corneal wound healing, ultimately suppressing TGF-β signaling. These findings hold significant implications for the development of novel therapeutic strategies targeting ocular diseases. By specifically modulating the activity of the ALK5/TGFBRI pathway, lumican-derived peptides may offer a targeted approach for alleviating the pathological consequences of dysregulated TGF-β signaling. Furthermore, this study highlights the potential of rationally designed peptides to selectively interfere with specific molecular pathways, providing a foundation for the development of precision therapies in the field of ophthalmology. Collectively, these studies highlight the multifaceted role of lumican in the eye, spanning from its contribution to the maintenance of corneal transparency and integrity to its involvement in ocular pathologies and wound healing processes. Further investigations into the molecular mechanisms underlying lumican’s functions and interactions with other ECM components are necessary to advance our understanding of its role in ocular health and disease, ultimately paving the way for the development of novel therapeutic strategies targeting lumican-related ocular pathologies.
Fibromodulin has potential therapeutic applications in tissue repair and regeneration, as it regulates collagen fibrillogenesis and exhibits anti-inflammatory properties [281,282]. Fibromodulin plays important roles in the modulation of various other biological processes besides collagen fibrillogenesis, including angiogenesis, TGF-β activity regulation, inflammatory mechanisms, apoptosis, differentiation of human fibroblasts into pluripotent cells and cancers [166]. Halasi et al. (2022) have recently shown that depletion of fibromodulin affects the expression of tight junctions proteins, contributing to epithelial barrier destruction, increased activated T-cells, plasmacytoid cells and type-I interferon production, and, thus, suggested to be a potential biomarker for severity of ulcerative colitis [283]. In the liver, fibromodulin contributes to the pathogenesis of fibrosis by regulating the fibrogenic phenotype of hepatic stellate cells [284]. The study by Zheng et al. (2016) reveals that high fibromodulin levels are correlated with decreased TGFβ1 expression and scarless repair, whereas low fibromodulin levels are correlated with increased TGFβ1 expression and scar formation [285,286]. Due to these diverse effects of fibromodulin on various tissues under different pathophysiological conditions, its therapeutic applications have been recently explored. A novel injectable fibromodulin-releasing hydrogel has been developed for promoting tendon healing [287]. Fibromodulin has been implied as a potential target for colorectal cancer treatment hence, its antagonist peptide, RP4, has been proposed to develop anti-colorectal cancer drugs [288]. Fibromodulin, administered via topical application, injection into the eye, or implantation in or on the eye, is currently being explored as an agent to reduce corneal scarring [289]. This patent application covers the use of 0.0001–5% fibromodulin for preventing corneal scarring, following injury, pathology and/or surgery [289]. Fibromodulin is also currently being explored to promote angiogenesis, improved tissue strength, and/or reduction of inflammation in the skin [290]. For such, fibromodulin can be administered via tissue implant or injection [290].
High myopia, a severe form of nearsightedness characterized by an excessive elongation of the eye’s axial length, represents a major public health concern due to its strong association with a heightened risk of developing vision-threatening ocular complications and the consequent impact on an individual’s quality of life. If an eye requires −6.0 diopters or more of lens correction, it is usually considered to have high myopia [291]. High myopia poses significant clinical challenges due to the increased risk of vision-threatening complications, such as retinal detachment, myopic macular degeneration, glaucoma, and cataract, which contribute to the global burden of visual impairment and necessitate timely intervention and management strategies to mitigate adverse outcomes. Mutations in genes coding opticin, lumican, fibromodulin, PRELP, and nyctalopin have all been associated with high myopia [292–296], and thus there is great potential in targeting these SLRPs for preventing myopia progression, however this remains a largely unexplored field of research.
Asporin is released by cardiac fibroblasts and attenuates TGFβ signaling during cardiac remodeling following heart failure [297]. Recently an asporin-mimic peptide capable of preventing transverse aortic constriction in mice was developed, demonstrating the potential of asporin-based therapies for treating various diseases [297]. Asporin also acts as an oncogene in some types of cancers, for example, breast [298,299], pancreatic [300], colorectal [301], gastric [302]and prostate [302] but as a tumor suppressor gene in triple-negative breast cancer [298]. In pancreatic [303] and gastric cancers [304], asporin was shown to interact directly with CD44, facilitating cancer cell migration and invasion in vitro and enhanced tumor metastasis in vivo. The CD44-asporin interaction involves the CD44/ERK/NF-κB/p65 axis to promote EMT, which has been proposed as a potential therapeutic target to decrease tumor migration and invasion. However, there is limited research on its direct applications in the eye. Asporin is expressed in various ocular tissues, including the cornea, retina, and optic nerve head [305]. Its interaction with TGFβ signaling and ECM organization suggests a potential role in modulating ocular tissue homeostasis and wound healing processes [299]. Further research is necessary to establish its specific functions and therapeutic applications in ocular diseases and disorders.
1.7. Future directions for SLRPs in medicine
Small leucine-rich proteoglycans (SLRPs) hold considerable promise in medicine, particularly due to their small size and multifaceted roles in modulating various biological processes, such as collagen fibrillogenesis, wound healing, inflammation, angiogenesis, and cellular signaling. Thus, SLRPs are attractive candidates for targeted therapeutic interventions. The ocular system has long been at the forefront of SLRP research and great progress has been made; however, future studies must focus on translating these studies into the clinic. Additionally, various functions of SLRPs in the ocular surface remain unexplored and warrant further investigation. The vital role of SLRPs, such as lumican, decorin, and fibromodulin, in maintaining corneal transparency, modulating wound healing, and regulating inflammation have been well established. Further research into the exact mechanism via which the SLRPs exert their various functions, in particular how they interact with growth factors and receptors, such as TGF-β, VEGF, and ALK5, will enable the generation of SLRP-derived peptides or mimetics with increased therapeutic and translational potential. Furthermore, elucidating the roles of less well-studied SLRPs, such as asporin, in ocular tissue homeostasis and wound healing processes could unveil new therapeutic strategies for ocular diseases. Beyond the eye, SLRPs are implicated in diverse physiological processes in various organs, including cartilage homeostasis, fibrosis, and cancer progression. Therefore, producing SLRP based therapies have the potential to treat various disorders, spanning various model systems. Thus, continued research into the various functional roles of SLRPs and the molecular mechanisms via which they exert their various tissue functions, in ocular and non-ocular systems, will undoubtedly expand the repertoire of therapeutic options, ultimately improving patient outcomes across a range of medical conditions.
Acknowledgements
This study was supported by start-up funds from the University of Houston for TFG, the National Institute of Health/National Eye Institute R01EY029289 and RO1EY033024 for VJCT.
Footnotes
Declaration of competing interest
Authors have no conflicts or financial interests related to this manuscript to disclose.
References
- [1].Gupta S, Buyank F, Sinha NR, Grant DG, Sinha PR, Iozzo RV, et al. Decorin regulates collagen fibrillogenesis during corneal wound healing in mouse in vivo. Exp Eye Res 2022;216:108933. 10.1016/j.exer.2022.108933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Schaefer L, Iozzo RV. Small leucine-rich proteoglycans, at the crossroad of cancer growth and inflammation. Curr Opin Genet Dev 2012;22:56–7. 10.1016/j.gde.2011.12.002. [DOI] [PubMed] [Google Scholar]
- [3].Simões RS, Soares-Jr JM, Simões MJ, Nader HB, Baracat MCP, Maciel GAR, et al. Small leucine-rich proteoglycans (SLRPs) in the endometrium of polycystic ovary syndrome women: a pilot study. J Ovarian Res 2017;10:54. 10.1186/s13048-017-0349-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Low SWY, Connor TB, Kassem IS, Costakos DM, Chaurasia SS. Small leucine-rich proteoglycans (SLRPs) in the retina. Int J Mol Sci 2021;22:7293. 10.3390/ijms22147293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Vuillermoz B, Khoruzhenko A, D’Onofrio M-F, Ramont L, Venteo L, Perreau C, et al. The small leucine-rich proteoglycan lumican inhibits melanoma progression. Exp Cell Res 2004;296:294–306. 10.1016/j.yexcr.2004.02.005. [DOI] [PubMed] [Google Scholar]
- [6].Appunni S, Anand V, Khandelwal M, Gupta N, Rubens M, Sharma A. Small Leucine Rich Proteoglycans (decorin, biglycan and lumican) in cancer. Clin Chim Acta 2019;491:1–7. 10.1016/j.cca.2019.01.003. [DOI] [PubMed] [Google Scholar]
- [7].Merline R, Schaefer RM, Schaefer L. The matricellular functions of small leucine-rich proteoglycans (SLRPs). J Cell Commun Signal 2009;3:323–35. 10.1007/s12079-009-0066-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Enkhbayar P, Kamiya M, Osaki M, Matsumoto T, Matsushima N. Structural principles of leucine-rich repeat (LRR) proteins. Proteins: Struct, Funct, Bioinf 2003;54:394–403. 10.1002/prot.10605. [DOI] [PubMed] [Google Scholar]
- [9].Kobe B, Deisenhofer J. A structural basis of the interactions between leucine-rich repeats and protein ligands. Nature 1995;374:183–6. 10.1038/374183a0. [DOI] [PubMed] [Google Scholar]
- [10].Kobe B, Deisenhofer J. The leucine-rich repeat: a versatile binding motif. Trends Biochem Sci 1994;19:415–21. 10.1016/0968-0004(94)90090-6. [DOI] [PubMed] [Google Scholar]
- [11].Buchanan SGStC Gay NJ. Structural and functional diversity in the leucine-rich repeat family of proteins. Prog Biophys Mol Biol 1996;65:1–44. 10.1016/S0079-6107(96)00003-X. [DOI] [PubMed] [Google Scholar]
- [12].Park H, Huxley-Jones J, Boot-Handford RP, Bishop PN, Attwood TK, Bella J. LRRCE: a leucine-rich repeat cysteine capping motif unique to the chordate lineage. BMC Genom 2008;9:599. 10.1186/1471-2164-9-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kajava AV. Structural diversity of leucine-rich repeat proteins 1 1Edited by F. Cohen. J Mol Biol 1998;277:519–27. 10.1006/jmbi.1998.1643. [DOI] [PubMed] [Google Scholar]
- [14].Kobe B. The leucine-rich repeat as a protein recognition motif. Curr Opin Struct Biol 2001;11:725–32. 10.1016/S0959-440X(01)00266-4. [DOI] [PubMed] [Google Scholar]
- [15].Kram V, Kilts TM, Bhattacharyya N, Li L, Young MF. Small leucine rich proteoglycans, a novel link to osteoclastogenesis. Sci Rep 2017;7:12627. 10.1038/s41598-017-12651-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chen S, Birk DE. The regulatory roles of small leucine-rich proteoglycans in extracellular matrix assembly. FEBS J 2013;280:2120–37. 10.1111/febs.12136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Iozzo RV. The biology of the small leucine-rich proteoglycans. J Biol Chem 1999;274:18843–6. 10.1074/jbc.274.27.18843. [DOI] [PubMed] [Google Scholar]
- [18].Zoeller JJ, Pimtong W, Corby H, Goldoni S, Iozzo AE, Owens RT, et al. A central role for decorin during vertebrate convergent extension. J Biol Chem 2009;284:11728–37. 10.1074/jbc.M808991200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Danielson KG, Baribault H, Holmes DF, Graham H, Kadler KE, Iozzo RV. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. JCB (J Cell Biol) 1997;136:729–43. 10.1083/jcb.136.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wegrowski Y, Pillarisetti J, Danielson KG, Suzuki S, Iozzo RV. The murine biglycan: complete cDNA cloning, genomic organization, promoter function, and expression. Genomics 1995;30:8–17. 10.1006/geno.1995.0002. [DOI] [PubMed] [Google Scholar]
- [21].Kalamajski S, Oldberg Å. The role of small leucine-rich proteoglycans in collagen fibrillogenesis. Matrix Biol 2010;29:248–53. 10.1016/j.matbio.2010.01.001. [DOI] [PubMed] [Google Scholar]
- [22].Iozzo RV. The family of the small leucine-rich proteoglycans: key regulators of matrix assembly and cellular growth. Crit Rev Biochem Mol Biol 1997;32:141–74. 10.3109/10409239709108551. [DOI] [PubMed] [Google Scholar]
- [23].Douglas T, Heinemann S, Bierbaum S, Scharnweber D, Worch H. Fibrillogenesis of collagen types I, II, and III with small leucine-rich proteoglycans decorin and biglycan. Biomacromolecules 2006;7:2388–93. 10.1021/bm0603746. [DOI] [PubMed] [Google Scholar]
- [24].Merline R, Nastase MV, Iozzo RV, Schaefer L. 3.5 Small leucine-rich proteoglycans: multifunctional signaling effectors. Extracellular Matrix: pathobiology and Signaling. DE GRUYTER; 2012. p. 185–96. 10.1515/9783110258776.185. [DOI] [Google Scholar]
- [25].Hill LJ, Moakes RJA, Vareechon C, Butt G, Ng A, Brock K, et al. Sustained release of decorin to the surface of the eye enables scarless corneal regeneration. NPJ Regen Med 2018;3:23. 10.1038/s41536-018-0061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Balne PK, Gupta S, Zhang J, Bristow D, Faubion M, Heil SD, et al. The functional role of decorin in corneal neovascularization in vivo. Exp Eye Res 2021;207:108610. 10.1016/j.exer.2021.108610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Iozzo RV, Moscatello DK, McQuillan DJ, Eichstetter I. Decorin is a biological ligand for the epidermal growth factor receptor. J Biol Chem 1999;274:4489–92. 10.1074/jbc.274.8.4489. [DOI] [PubMed] [Google Scholar]
- [28].Horváth Z, Kovalszky I, Fullár A, Kiss K, Schaff Z, Iozzo RV, et al. Decorin deficiency promotes hepatic carcinogenesis. Matrix Biol 2014;35:194–205. 10.1016/j.matbio.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Morcavallo A, Buraschi S, Xu S-Q, Belfiore A, Schaefer L, Iozzo RV, et al. Decorin differentially modulates the activity of insulin receptor isoform A ligands. Matrix Biol 2014;35:82–90. 10.1016/j.matbio.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Goldoni S, Humphries A, Nyström A, Sattar S, Owens RT, McQuillan DJ, et al. Decorin is a novel antagonistic ligand of the Met receptor. JCB (J Cell Biol) 2009;185:743–54. 10.1083/jcb.200901129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Merline R, Moreth K, Beckmann J, Nastase MV, Zeng-Brouwers J, Tralhão JG, et al. Signaling by the matrix proteoglycan decorin controls inflammation and cancer through PDCD4 and MicroRNA-21. Sci Signal 2011;4. 10.1126/scisignal.2001868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Neill T, Schaefer L, Iozzo RV. Decorin as a multivalent therapeutic agent against cancer. Adv Drug Deliv Rev 2016;97:174–85. 10.1016/j.addr.2015.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Iozzo RV. Proteoglycans and neoplasia. Cancer Metastasis Review 1988;7:39–50. 10.1007/BF00048277. [DOI] [PubMed] [Google Scholar]
- [34].Goldoni S, Iozzo RV. Tumor microenvironment: modulation by decorin and related molecules harboring leucine-rich tandem motifs. Int J Cancer 2008;123:2473–9. 10.1002/ijc.23930. [DOI] [PubMed] [Google Scholar]
- [35].Iozzo RV, Schaefer L. Proteoglycans in health and disease: novel regulatory signaling mechanisms evoked by the small leucine-rich proteoglycans. FEBS J 2010;277:3864–75. 10.1111/j.1742-4658.2010.07797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Goldoni S, Owens RT, McQuillan DJ, Shriver Z, Sasisekharan R, Birk DE, et al. Biologically active decorin is a monomer in solution. J Biol Chem 2004;279:6606–12. 10.1074/jbc.M310342200. [DOI] [PubMed] [Google Scholar]
- [37].Kao WW-Y, Funderburgh JL, Xia Y, Liu C-Y, Conrad GW. Focus on molecules: lumican. Exp Eye Res 2006;82:3–4. 10.1016/j.exer.2005.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Matsushima N, Miyashita H, Kretsinger RH. Sequence features, structure, ligand interaction, and diseases in small leucine rich repeat proteoglycans. J Cell Commun Signal 2021;15:519–31. 10.1007/s12079-021-00616-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Nikitovic D, Tzanakakis G. Small leucine-rich proteoglycans regulate cancer cell growth. In: Apoptosis, and associated inflammation; 2022. p. 1–21. 10.1007/978-3-030-99708-3_1. [DOI] [Google Scholar]
- [40].Pietraszek-Gremplewicz K, Karamanou K, Niang A, Dauchez M, Belloy N, Maquart F-X, et al. Small leucine-rich proteoglycans and matrix metalloproteinase-14: key partners? Matrix Biol 2019;75(76):271–85. 10.1016/j.matbio.2017.12.006. [DOI] [PubMed] [Google Scholar]
- [41].Schaefer L, Iozzo RV. Biological functions of the small leucine-rich proteoglycans: from genetics to signal transduction. J Biol Chem 2008;283:21305–9. 10.1074/jbc.R800020200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Rivet R, Rao RM, Nizet P, Belloy N, Huber L, Dauchez M, et al. Differential MMP-14 targeting by biglycan, decorin, fibromodulin, and lumican unraveled by in silico approach. Am J Physiol Cell Physiol 2023;324:C353–65. 10.1152/ajpcell.00429.2022. [DOI] [PubMed] [Google Scholar]
- [43].Puri S, Coulson-Thomas YM, Gesteira TF, Coulson-Thomas VJ. Distribution and function of glycosaminoglycans and proteoglycans in the development, homeostasis and pathology of the ocular surface. Front Cell Dev Biol 2020;8. 10.3389/fcell.2020.00731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kajava AV, Vassart G, Wodak SJ. Modeling of the three-dimensional structure of proteins with the typical leucine-rich repeats. Structure 1995;3:867–77. 10.1016/S0969-2126(01)00222-2. [DOI] [PubMed] [Google Scholar]
- [45].Hindle KL, Bella J, Lovell SC. Quantitative analysis and prediction of curvature in leucine-rich repeat proteins. Proteins: Struct, Funct, Bioinf 2009;77:342–58. 10.1002/prot.22440. [DOI] [PubMed] [Google Scholar]
- [46].Morita J, Kato K, Nakane T, Kondo Y, Fukuda H, Nishimasu H, et al. Crystal structure of the plant receptor-like kinase TDR in complex with the TDIF peptide. Nat Commun 2016;7:12383. 10.1038/ncomms12383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Bella J, Hindle KL, McEwan PA, Lovell SC. The leucine-rich repeat structure. Cell Mol Life Sci 2008;65:2307–33. 10.1007/s00018-008-8019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Liu P, Hu Z, Zhou B, Liu S, Chai J. Crystal structure of an LRR protein with two solenoids. Cell Res 2013;23:303–5. 10.1038/cr.2012.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Jackson VA, Mehmood S, Chavent M, Roversi P, Carrasquero M, del Toro D, et al. Super-complexes of adhesion GPCRs and neural guidance receptors. Nat Commun 2016;7:11184. 10.1038/ncomms11184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Sanders P, Young S, Sanders J, Kabelis K, Baker S, Sullivan A, et al. Crystal structure of the TSH receptor bound to a blocking type TSHR autoantibody. J Mol Endocrinol 2011. 10.1530/JME-10-0127. [DOI] [PubMed] [Google Scholar]
- [51].McEwan PA, Andrews RK, Emsley J. Glycoprotein Ibα inhibitor complex structure reveals a combined steric and allosteric mechanism of von Willebrand factor antagonism. Blood 2009;114:4883–5. 10.1182/blood-2009-05-224170. [DOI] [PubMed] [Google Scholar]
- [52].Xu K, Xu Y, Rajashankar KR, Robev D, Nikolov DB. Crystal structures of Lgr4 and its complex with R-spondin1. Structure 2013;21:1683–9. 10.1016/j.str.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Yoon S, Hong M, Wilson IA. An unusual dimeric structure and assembly for TLR4 regulator RP105–MD-1. Nat Struct Mol Biol 2011;18:1028–35. 10.1038/nsmb.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Deng L, Velikovsky CA, Xu G, Iyer LM, Tasumi S, Kerzic MC, et al. A structural basis for antigen recognition by the T cell-like lymphocytes of sea lamprey. Proc Natl Acad Sci USA 2010;107:13408–13. 10.1073/pnas.1005475107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Velikovsky CA, Deng L, Tasumi S, Iyer LM, Kerzic MC, Aravind L, et al. Structure of a lamprey variable lymphocyte receptor in complex with a protein antigen. Nat Struct Mol Biol 2009;16:725–30. 10.1038/nsmb.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Truhlar SME, Komives EA. LRR domain folding: just put a cap on it. Structure 2008;16:655–7. 10.1016/j.str.2008.04.002. [DOI] [PubMed] [Google Scholar]
- [57].Goldoni S, Owens RT, McQuillan DJ, Shriver Z, Sasisekharan R, Birk DE, et al. Biologically active decorin is a monomer in solution. J Biol Chem 2004;279:6606–12. 10.1074/jbc.M310342200. [DOI] [PubMed] [Google Scholar]
- [58].Henry SP, Takanosu M, Boyd TC, Mayne PM, Eberspaecher H, Zhou W, et al. Expression pattern and gene characterization ofAsporin. J Biol Chem 2001;276:12212–21. 10.1074/jbc.M011290200. [DOI] [PubMed] [Google Scholar]
- [59].Oritani K, Kincade PW. Lymphopoiesis and matrix glycoprotein SC1/ECM2. Leuk Lymphoma 1998;32:1–7. 10.3109/10428199809059241. [DOI] [PubMed] [Google Scholar]
- [60].Hynes RO, Naba A. Overview of the matrisome–an inventory of extracellular matrix constituents and functions. Cold Spring Harbor Perspect Biol 2012;4. 10.1101/cshperspect.a004903. a004903–a004903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Arends F, Nowald C, Pflieger K, Boettcher K, Zahler S, Lieleg O. The biophysical properties of basal lamina gels depend on the biochemical composition of the gel. PLoS One 2015;10:e0118090. 10.1371/journal.pone.0118090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Chakravarti S, Stallings RL, SundarRaj N, Cornuet PK, Hassell JR. Primary structure of human lumican (keratan sulfate proteoglycan) and localization of the gene (LUM) to chromosome 12q21.3-q22. Genomics 1995;27:481–8. 10.1006/geno.1995.1080. [DOI] [PubMed] [Google Scholar]
- [63].Karamanou K, Perrot G, Maquart F-X, Brézillon S. Lumican as a multivalent effector in wound healing. Adv Drug Deliv Rev 2018;129:344–51. 10.1016/j.addr.2018.02.011. [DOI] [PubMed] [Google Scholar]
- [64].Funderburgh JL. MINI REVIEW Keratan sulfate: structure, biosynthesis, and function. Glycobiology 2000;10:951–8. 10.1093/glycob/10.10.951. [DOI] [PubMed] [Google Scholar]
- [65].Melching LI, Roughley PJ. Modulation of keratan sulfate synthesis on lumican by the action of cytokines on human articular chondrocytes. Matrix Biol 1999;18:381–90. 10.1016/S0945-053X(99)00033-5. [DOI] [PubMed] [Google Scholar]
- [66].Jensen MM, Karring H. The origins and developments of sulfation-prone tyrosine-rich and acidic N- and C-terminal extensions of class ll and lll small leucine-rich repeat proteins shed light on connective tissue evolution in vertebrates. BMC Evol Biol 2020;20:73. 10.1186/s12862-020-01634-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Maiti G, Frikeche J, Lam CY-M, Biswas A, Shinde V, Samanovic M, et al. Matrix lumican endocytosed by immune cells controls receptor ligand trafficking to promote TLR4 and restrict TLR9 in sepsis. Proc Natl Acad Sci USA 2021;118. 10.1073/pnas.2100999118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Yamanaka O, Yuan Y, Coulson-Thomas VJ, Gesteira TF, Call MK, Zhang Y, et al. Lumican binds ALK5 to promote epithelium wound healing. PLoS One 2013;8:e82730. 10.1371/journal.pone.0082730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Gesteira TF, Coulson-Thomas VJ, Yuan Y, Zhang J, Nader HB, Kao WW-Y. Lumican peptides: rational design targeting ALK5/TGFBRI. Sci Rep 2017;7:42057. 10.1038/srep42057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Kalamajski S, Oldberg Å. Fibromodulin binds collagen type I via glu-353 and lys-355 in leucine-rich repeat 11. J Biol Chem 2007;282:26740–5. 10.1074/jbc.M704026200. [DOI] [PubMed] [Google Scholar]
- [71].Kalamajski S, Oldberg Å. Homologous sequence in lumican and fibromodulin leucine-rich repeat 5–7 competes for collagen binding. J Biol Chem 2009;284:534–9. 10.1074/jbc.M805721200. [DOI] [PubMed] [Google Scholar]
- [72].Paracuellos P, Kalamajski S, Bonna A, Bihan D, Farndale RW, Hohenester E. Structural and functional analysis of two small leucine-rich repeat proteoglycans, fibromodulin and chondroadherin. Matrix Biol 2017;63:106–16. 10.1016/j.matbio.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Tashima T, Nagatoishi S, Caaveiro JMM, Nakakido M, Sagara H, Kusano-Arai O, et al. Molecular basis for governing the morphology of type-I collagen fibrils by Osteomodulin. Commun Biol 2018;1:33. 10.1038/s42003-018-0038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Lin W, Zhu X, Gao L, Mao M, Gao D, Huang Z. Osteomodulin positively regulates osteogenesis through interaction with BMP2. Cell Death Dis 2021;12:147. 10.1038/s41419-021-03404-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Matsushima N, Miyashita H, Kretsinger RH. Sequence features, structure, ligand interaction, and diseases in small leucine rich repeat proteoglycans. J Cell Commun Signal 2021;15:519–31. 10.1007/s12079-021-00616-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Ross MD, Bruggeman LA, Hanss B, Sunamoto M, Marras D, Klotman ME, et al. Podocan, a novel small leucine-rich repeat protein expressed in the sclerotic glomerular lesion of experimental HIV-associated nephropathy. J Biol Chem 2003;278:33248–55. 10.1074/jbc.M301299200. [DOI] [PubMed] [Google Scholar]
- [77].Kanwar YS, Linker A, Farquhar MG. Increased permeability of the glomerular basement membrane to ferritin after removal of glycosaminoglycans (heparan sulfate) by enzyme digestion. JCB (J Cell Biol) 1980;86:688–93. 10.1083/jcb.86.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Chen L, Kashina A. Post-translational modifications of the protein termini. Front Cell Dev Biol 2021;9. 10.3389/fcell.2021.719590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Pieroni L, Iavarone F, Olianas A, Greco V, Desiderio C, Martelli C, et al. Enrichments of post-translational modifications in proteomic studies. J Separ Sci 2020;43:313–36. 10.1002/jssc.201900804. [DOI] [PubMed] [Google Scholar]
- [80].Zappia J, Joiret M, Sanchez C, Lambert C, Geris L, Muller M, et al. From translation to protein degradation as mechanisms for regulating biological functions: a review on the SLRP family in skeletal tissues. Biomolecules 2020;10:80. 10.3390/biom10010080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].An HJ, Froehlich JW, Lebrilla CB. Determination of glycosylation sites and site-specific heterogeneity in glycoproteins. Curr Opin Chem Biol 2009;13:421–6. 10.1016/j.cbpa.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Reily C, Stewart TJ, Renfrow MB, Novak J. Glycosylation in health and disease. Nat Rev Nephrol 2019;15:346–66. 10.1038/s41581-019-0129-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Frikeche J, Maiti G, Chakravarti S. Small leucine-rich repeat proteoglycans in corneal inflammation and wound healing. Exp Eye Res 2016;151:142–9. 10.1016/J.EXER.2016.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].El Masri R, Seffouh A, Lortat-Jacob H, Vivès RR. The “in and out” of glucosamine 6-O-sulfation: the 6th sense of heparan sulfate. Glycoconj J 2017;34:285–98. 10.1007/s10719-016-9736-5. [DOI] [PubMed] [Google Scholar]
- [85].El Masri R, Seffouh A, Roelants C, Seffouh I, Gout E, Pérard J, et al. Extracellular endosulfatase Sulf-2 harbors a chondroitin/dermatan sulfate chain that modulates its enzyme activity. Cell Rep 2022;38:110516. 10.1016/j.celrep.2022.110516. [DOI] [PubMed] [Google Scholar]
- [86].Ajit Varki RDCJDEPSGWHMADMTKNHPJHPRLS PHS. Essentials of glycobiology. In: Cold spring harbor. fourth ed. (NY): Cold Spring Harbor Laboratory Press; 2022. [Google Scholar]
- [87].Frikeche J, Maiti G, Chakravarti S. Small leucine-rich repeat proteoglycans in corneal inflammation and wound healing. Exp Eye Res 2016;151:142–9. 10.1016/j.exer.2016.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Quantock AJ, Young RD, Akama TO. Structural and biochemical aspects of keratan sulphate in the cornea. Cell Mol Life Sci 2010;67:891–906. 10.1007/s00018-009-0228-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Funderburgh JL. Keratan sulfate biosynthesis. IUBMB Life 2002;54:187–94. 10.1080/15216540214932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Krishnan P, Hocking AM, Scholtz JM, Pace CN, Holik KK, McQuillan DJ. Distinct secondary structures of the leucine-rich repeat proteoglycans decorin and biglycan. J Biol Chem 1999;274:10945–50. 10.1074/jbc.274.16.10945. [DOI] [PubMed] [Google Scholar]
- [91].Seo N-S, Hocking AM, Höök M, McQuillan DJ. Decorin core protein secretion is regulated by N-linked oligosaccharide and glycosaminoglycan additions. J Biol Chem 2005;280:42774–84. 10.1074/jbc.M511531200. [DOI] [PubMed] [Google Scholar]
- [92].Raspanti M, Viola M, Forlino A, Tenni R, Gruppi C, Tira ME. Glycosaminoglycans show a specific periodic interaction with type I collagen fibrils. J Struct Biol 2008;164:134–9. 10.1016/j.jsb.2008.07.001. [DOI] [PubMed] [Google Scholar]
- [93].Lewis PN, Pinali C, Young RD, Meek KM, Quantock AJ, Knupp C. Structural interactions between collagen and proteoglycans are elucidated by three-dimensional electron tomography of bovine cornea. Structure 2010;18:239–45. 10.1016/j.str.2009.11.013. [DOI] [PubMed] [Google Scholar]
- [94].Parfitt GJ, Pinali C, Young RD, Quantock AJ, Knupp C. Three-dimensional reconstruction of collagen–proteoglycan interactions in the mouse corneal stroma by electron tomography. J Struct Biol 2010;170:392–7. 10.1016/j.jsb.2010.01.019. [DOI] [PubMed] [Google Scholar]
- [95].Stamov DR, Müller A, Wegrowski Y, Brezillon S, Franz CM. Quantitative analysis of type I collagen fibril regulation by lumican and decorin using AFM. J Struct Biol 2013;183:394–403. 10.1016/j.jsb.2013.05.022. [DOI] [PubMed] [Google Scholar]
- [96].Tatara Y, Suto S, Itoh K. Novel roles of glycosaminoglycans in the degradation of type I collagen by cathepsin K. Glycobiology 2017;27:1089–98. 10.1093/glycob/cwx083. [DOI] [PubMed] [Google Scholar]
- [97].Brockhausen I, Elimova E, Woodward AM, Argüeso P. Glycosylation pathways of human corneal and conjunctival epithelial cell mucins. Carbohydr Res 2018;470:50–6. 10.1016/j.carres.2018.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Baeuerle PA, Huttner WB. Tyrosine sulfation is a trans-Golgi-specific protein modification. JCB (J Cell Biol) 1987;105:2655–64. 10.1083/jcb.105.6.2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Woods AS, Wang H-YJ, Jackson SN. Sulfation, the up-and-coming post-translational modification: its role and mechanism in Protein–Protein interaction. J Proteome Res 2007;6:1176–82. 10.1021/pr060529g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Niehrs C, Beißwanger R, Huttner WB. Protein tyrosine sulfation, 1993 — an update. Chem Biol Interact 1994;92:257–71. 10.1016/0009-2797(94)90068-X. [DOI] [PubMed] [Google Scholar]
- [101].Yang Y-S, Wang C-C, Chen B-H, Hou Y-H, Hung K-S, Mao Y-C. Tyrosine sulfation as a protein post-translational modification. Molecules 2015;20:2138–64. 10.3390/molecules20022138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Costagliola S Tyrosine sulfation is required for agonist recognition by glycoprotein hormone receptors. EMBO J 2002;21:504–13. 10.1093/emboj/21.4.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Nlend M-C, Cauvi D, Venot N, Chabaud O. Sulfated tyrosines of thyroglobulin are involved in thyroid hormone synthesis. Biochem Biophys Res Commun 1999;262:193–7. 10.1006/bbrc.1999.1173. [DOI] [PubMed] [Google Scholar]
- [104].Farzan M, Mirzabekov T, Kolchinsky P, Wyatt R, Cayabyab M, Gerard NP, et al. Tyrosine sulfation of the amino terminus of CCR5 facilitates HIV-1 entry. Cell 1999;96:667–76. 10.1016/S0092-8674(00)80577-2. [DOI] [PubMed] [Google Scholar]
- [105].Bannert N, Craig S, Farzan M, Sogah D, Santo NV, Choe H, et al. Sialylated O-glycans and sulfated tyrosines in the NH2-terminal domain of CC chemokine receptor 5 contribute to high affinity binding of chemokines. J Exp Med 2001;194:1661–74. 10.1084/jem.194.11.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Farzan M, Schnitzler CE, Vasilieva N, Leung D, Kuhn J, Gerard C, et al. Sulfated tyrosines contribute to the formation of the C5a docking site of the human C5a anaphylatoxin receptor. J Exp Med 2001;193:1059–66. 10.1084/jem.193.9.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Bernimoulin MP, Zeng X-L, Abbal C, Giraud S, Martinez M, Michielin O, et al. Molecular basis of leukocyte rolling on PSGL-1. J Biol Chem 2003;278:37–47. 10.1074/jbc.M204360200. [DOI] [PubMed] [Google Scholar]
- [108].Leppänen A, White SP, Helin J, McEver RP, Cummings RD. Binding of glycosulfopeptides to P-selectin requires stereospecific contributions of individual tyrosine sulfate and sugar residues. J Biol Chem 2000;275:39569–78. 10.1074/jbc.M005005200. [DOI] [PubMed] [Google Scholar]
- [109].Wilkins PP, Moore KL, McEver RP, Cummings RD. Tyrosine sulfation of P-selectin glycoprotein ligand-1 is required for high affinity binding to P-selectin. J Biol Chem 1995;270:22677–80. 10.1074/jbc.270.39.22677. [DOI] [PubMed] [Google Scholar]
- [110].Önnerfjord P, Heathfield TF, Heinegård D. Identification of tyrosine sulfation in extracellular leucine-rich repeat proteins using mass spectrometry. J Biol Chem 2004;279:26–33. 10.1074/jbc.M308689200. [DOI] [PubMed] [Google Scholar]
- [111].Tillgren V, Önnerfjord P, Haglund L, Heinegård D. The tyrosine sulfate-rich domains of the LRR proteins fibromodulin and osteoadherin bind motifs of basic clusters in a variety of heparin-binding proteins, including bioactive factors. J Biol Chem 2009;284:28543–53. 10.1074/jbc.M109.047076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Tillgren V, Mörgelin M, Önnerfjord P, Kalamajski S, Aspberg A. The tyrosine sulfate domain of fibromodulin binds collagen and enhances fibril formation. J Biol Chem 2016;291:23744–55. 10.1074/jbc.M116.730325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Kanan Y, Siefert JC, Kinter M, Al-Ubaidi MR. Complement factor H, vitronectin, and opticin are tyrosine-sulfated proteins of the retinal pigment epithelium. PLoS One 2014;9:e105409. 10.1371/journal.pone.0105409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Le Goff MM, Lu H, Ugarte M, Henry S, Takanosu M, Mayne R, et al. The vitreous glycoprotein opticin inhibits preretinal neovascularization. Investig Opthalmol Visual Sci 2012;53:228. 10.1167/iovs.11-8514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Sanders EJ, Walter MA, Parker E, Arámburo C, Harvey S. Opticin binds retinal growth hormone in the embryonic vitreous. Investig Opthalmol Visual Sci 2003;44:5404. 10.1167/iovs.03-0500. [DOI] [PubMed] [Google Scholar]
- [116].Le Goff MM, Sutton MJ, Slevin M, Latif A, Humphries MJ, Bishop PN. Opticin exerts its anti-angiogenic activity by regulating extracellular matrix adhesiveness. J Biol Chem 2012;287:28027–36. 10.1074/jbc.M111.331157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Imai K, Hiramatsu A, Fukushima D, Pierschbacher MD, Okada Y. Degradation of decorin by matrix metalloproteinases: identification of the cleavage sites, kinetic analyses and transforming growth factor- β 1 release. Biochem J 1997;322:809–14. 10.1042/bj3220809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Li Y, Aoki T, Mori Y, Ahmad M, Miyamori H, Takino T, et al. Cleavage of lumican by membrane-type matrix metalloproteinase-1 abrogates this proteoglycan-mediated suppression of tumor cell colony formation in soft agar. Cancer Res 2004;64:7058–64. 10.1158/0008-5472.CAN-04-1038. [DOI] [PubMed] [Google Scholar]
- [119].Zhang L, Yang M, Yang D, Cavey G, Davidson P, Gibson G. Molecular interactions of MMP-13 C-terminal domain with chondrocyte proteins. Connect Tissue Res 2010;51:230–9. 10.3109/03008200903288902. [DOI] [PubMed] [Google Scholar]
- [120].Monfort J, Tardif G, Roughley P, Reboul P, Boileau C, Bishop PN, et al. Identification of opticin, a member of the small leucine-rich repeat proteoglycan family, in human articular tissues: a novel target for MMP-13 in osteoarthritis. Osteoarthritis Cartilage 2008;16:749–55. 10.1016/j.joca.2007.11.007. [DOI] [PubMed] [Google Scholar]
- [121].Heathfield TF, Önnerfjord P, Dahlberg L, Heinegård D. Cleavage of fibromodulin in cartilage explants involves removal of the N-terminal tyrosine sulfate-rich region by proteolysis at a site that is sensitive to matrix metalloproteinase-13. J Biol Chem 2004;279:6286–95. 10.1074/jbc.M307765200. [DOI] [PubMed] [Google Scholar]
- [122].Monfort J, Tardif G, Reboul P, Mineau F, Roughley P, Pelletier J-P, et al. Degradation of small leucine-rich repeat proteoglycans by matrix metalloprotease-13: identification of a new biglycan cleavage site. Arthritis Res Ther 2006;8:R26. 10.1186/ar1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Tío L, Martel-Pelletier J, Pelletier J-P, Bishop PN, Roughley P, Farran A, et al. Characterization of opticin digestion by proteases involved in osteoarthritis development. Joint Bone Spine 2014;81:137–41. 10.1016/j.jbspin.2013.05.007. [DOI] [PubMed] [Google Scholar]
- [124].Genovese F, Barascuk N, Larsen L, Larsen MR, Nawrocki A, Li Y, et al. Biglycan fragmentation in pathologies associated with extracellular matrix remodeling by matrix metalloproteinases. Fibrogenesis Tissue Repair 2013;6:9. 10.1186/1755-1536-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Melching LI, Fisher WD, Lee ER, Mort JS, Roughley PJ. The cleavage of biglycan by aggrecanases. Osteoarthritis Cartilage 2006;14:1147–54. 10.1016/j.joca.2006.05.014. [DOI] [PubMed] [Google Scholar]
- [126].Boivin WA, Shackleford M, Vanden Hoek A, Zhao H, Hackett TL, Knight DA, et al. Granzyme B cleaves decorin, biglycan and soluble betaglycan, releasing active transforming growth factor-β1. PLoS One 2012;7:e33163. 10.1371/journal.pone.0033163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Akhatib B, Önnerfjord P, Gawri R, Ouellet J, Jarzem P, Heinegård D, et al. Chondroadherin fragmentation mediated by the protease HTRA1 distinguishes human intervertebral disc degeneration from normal aging. J Biol Chem 2013;288:19280. 10.1074/jbc.M112.443010.-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Frevert CW, Felgenhauer J, Wygrecka M, Nastase MV, Schaefer L. Danger-associated molecular patterns derived from the extracellular matrix provide temporal control of innate immunity. J Histochem Cytochem 2018;66:213–27. 10.1369/0022155417740880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Hausser H, Hoppe W, Rauch U, Kresse H. Endocytosis of a small dermatan sulphate proteoglycan. Identification of binding proteins. Biochem J 1989;263:137–42. 10.1042/bj2630137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Sofeu Feugaing DD, Kresse H, Greb RR, Götte M. A novel 110-kDa receptor protein is involved in endocytic uptake of decorin by human skin fibroblasts. Sci World J 2006;6:35–52. 10.1100/tsw.2006.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Schönherr E, Sunderkötter C, Iozzo RV, Schaefer L. Decorin, a novel player in the insulin-like growth factor system. J Biol Chem 2005;280:15767–72. 10.1074/jbc.M500451200. [DOI] [PubMed] [Google Scholar]
- [132].Santiago-García J, Kodama T, Pitas RE. The class A scavenger receptor binds to proteoglycans and mediates adhesion of macrophages to the extracellular matrix. J Biol Chem 2003;278:6942–6. 10.1074/jbc.M208358200. [DOI] [PubMed] [Google Scholar]
- [133].Hausser H, Kresse H. Decorin endocytosis: structural features of heparin and heparan sulphate oligosaccharides interfering with receptor binding and endocytosis. Biochem J 1999;344:827. 10.1042/0264-6021:3440827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Schmidt G, Hausser H, Kresse H. Extracellular accumulation of small dermatan sulphate proteoglycan II by interference with the secretion-recapture pathway. Biochem J 1990;266:591–5. [PMC free article] [PubMed] [Google Scholar]
- [135].Feugaing D, Tammi R, Echtermeyer F, Stenmark H, Kresse H, Smollich M, et al. Endocytosis of the dermatan sulfate proteoglycan decorin utilizes multiple pathways and is modulated by epidermal growth factor receptor signaling. Biochimie 2007;89:637–57. 10.1016/j.biochi.2006.12.012. [DOI] [PubMed] [Google Scholar]
- [136].Coulson-Thomas VJ, Caterson B, Kao WW-Y. Transplantation of human umbilical mesenchymal stem cells cures the corneal defects of mucopolysaccharidosis VII mice. Stem Cell 2013;31:2116–26. 10.1002/stem.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Ashworth JL, Biswas S, Wraith E, Lloyd IC. The ocular features of the mucopolysaccharidoses. Eye 2006;20:553–63. 10.1038/sj.eye.6701921. [DOI] [PubMed] [Google Scholar]
- [138].Chakravarti S, Zhang G, Chervoneva I, Roberts L, Birk DE. Collagen fibril assembly during postnatal development and dysfunctional regulation in the lumican-deficient murine cornea. Dev Dynam 2006;235:2493–506. 10.1002/dvdy.20868. [DOI] [PubMed] [Google Scholar]
- [139].Zheng Z, Nguyen C, Zhang X, Khorasani H, Wang JZ, Zara JN, et al. Delayed wound closure in fibromodulin-deficient mice is associated with increased TGF-β3 signaling. J Invest Dermatol 2011;131:769–78. 10.1038/jid.2010.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Chen S, Oldberg A, Chakravarti S, Birk DE. Fibromodulin regulates collagen fibrillogenesis during peripheral corneal development. Dev Dynam 2010;239:844–54. 10.1002/dvdy.22216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Chakravarti S, Paul J, Roberts L, Chervoneva I, Oldberg A, Birk DE. Ocular and scleral alterations in gene-targeted lumican-fibromodulin double-null mice. Investig Opthalmol Visual Sci 2003;44:2422. 10.1167/iovs.02-0783. [DOI] [PubMed] [Google Scholar]
- [142].Carlson EC, Liu C-Y, Chikama T, Hayashi Y, Kao CW-C, Birk DE, et al. Keratocan, a cornea-specific keratan sulfate proteoglycan, is regulatedby lumican. J Biol Chem 2005;280:25541–7. 10.1074/jbc.M500249200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Saika S, Shiraishi A, Saika S, Liu C-Y, Funderburgh JL, Kao CW-C, et al. Role of lumican in the corneal epithelium during wound healing. J Biol Chem 2000;275:2607–12. 10.1074/jbc.275.4.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Vij N, Roberts L, Joyce S, Chakravarti S. Lumican regulates corneal inflammatory responses by modulating fas-fas ligand signaling. Investig Opthalmol Visual Sci 2005;46:88. 10.1167/iovs.04-0833. [DOI] [PubMed] [Google Scholar]
- [145].Yeh L-K, Chen W-L, Li W, Espana EM, Ouyang J, Kawakita T, et al. Soluble lumican glycoprotein purified from human amniotic membrane promotes corneal epithelial wound healing. Investig Opthalmol Visual Sci 2005;46:479. 10.1167/iovs.04-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Seomun Y, Joo C-K. Lumican induces human corneal epithelial cell migration and integrin expression via ERK 1/2 signaling. Biochem Biophys Res Commun 2008;372:221–5. 10.1016/j.bbrc.2008.05.014. [DOI] [PubMed] [Google Scholar]
- [147].Moreth K, Frey H, Hubo M, Zeng-Brouwers J, Nastase M-V, Hsieh LT-H, et al. Biglycan-triggered TLR-2- and TLR-4-signaling exacerbates the pathophysiology of ischemic acute kidney injury. Matrix Biol 2014;35:143–51. 10.1016/j.matbio.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Barreto G, Senturk B, Colombo L, Brück O, Neidenbach P, Salzmann G, et al. Lumican is upregulated in osteoarthritis and contributes to TLR4-induced pro-inflammatory activation of cartilage degradation and macrophage polarization. Osteoarthritis Cartilage 2020;28:92–101. 10.1016/j.joca.2019.10.011. [DOI] [PubMed] [Google Scholar]
- [149].Lee S, Bowrin K, Hamad AR, Chakravarti S. Extracellular matrix lumican deposited on the surface of neutrophils promotes migration by binding to β2 integrin. J Biol Chem 2009;284:23662–9. 10.1074/jbc.M109.026229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Shao H, Scott S-G, Nakata C, Hamad AR, Chakravarti S. Extracellular matrix protein lumican promotes clearance and resolution of Pseudomonas aeruginosa keratitis in a mouse model. PLoS One 2013;8:e54765. 10.1371/journal.pone.0054765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Barbariga M, Vallone F, Mosca E, Bignami F, Magagnotti C, Fonteyne P, et al. The role of extracellular matrix in mouse and human corneal neovascularization. Sci Rep 2019;9:14272. 10.1038/s41598-019-50718-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Niewiarowska J, Brézillon S, Sacewicz-Hofman I, Bednarek R, Maquart F-X, Malinowski M, et al. Lumican inhibits angiogenesis by interfering with α2β1 receptor activity and downregulating MMP-14 expression. Thromb Res 2011;128:452–7. 10.1016/j.thromres.2011.06.011. [DOI] [PubMed] [Google Scholar]
- [153].Hayashida Y, Akama TO, Beecher N, Lewis P, Young RD, Meek KM, et al. Matrix morphogenesis in cornea is mediated by the modification of keratan sulfate by GlcNAc 6- O -sulfotransferase. Proc Natl Acad Sci USA 2006;103:13333–8. 10.1073/pnas.0605441103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Hasegawa N, Torii T, Kato T, Miyajima H, Furuhata A, Nakayasu K, et al. Decreased GlcNAc 6-O-sulfotransferase activity in the cornea with macular corneal dystrophy. Invest Ophthalmol Vis Sci 2000;41:3670–7. [PubMed] [Google Scholar]
- [155].Cornuet PK, Blochberger TC, Hassell JR. Molecular polymorphism of lumican during corneal development. Invest Ophthalmol Vis Sci 1994;35:870–7. [PubMed] [Google Scholar]
- [156].Dunlevy JR, Beales MP, Berryhill BL, Cornuet PK, Hassell JR. Expression of the keratan sulfate proteoglycans lumican, keratocan and osteoglycin/mimecan during chick corneal development. Exp Eye Res 2000;70:349–62. 10.1006/exer.1999.0789. [DOI] [PubMed] [Google Scholar]
- [157].Ying S, Shiraishi A, Kao CW-C, Converse RL, Funderburgh JL, Swiergiel J, et al. Characterization and expression of the mouse lumican gene. J Biol Chem 1997;272:30306–13. 10.1074/jbc.272.48.30306. [DOI] [PubMed] [Google Scholar]
- [158].Waddington R, Roberts H, Sugars R, Schönherr E. Differential roles for small leucine-rich proteoglycans in bone formation. Eur Cell Mater 2003;6:12–21. 10.22203/eCM.v006a02. [DOI] [PubMed] [Google Scholar]
- [159].Johnstone B, Markopoulos M, Neame P, Caterson B. Identification and characterization of glycanated and non-glycanated forms of biglycan and decorin in the human intervertebral disc. Biochem J 1993;292:661–6. 10.1042/bj2920661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Kao WW-Y, Liu C-Y. Roles of lumican and keratocan on corneal transparency. Glycoconj J 2002;19:275–85. 10.1023/A:1025396316169. [DOI] [PubMed] [Google Scholar]
- [161].Zhang Y, Kao WW-Y, Hayashi Y, Zhang L, Call M, Dong F, et al. Generation and characterization of a novel mouse line, keratocan-rtTA (kera RT), for corneal stroma and tendon research. Investig Opthalmol Visual Sci 2017;58:4800. 10.1167/iovs.17-22661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Pellegata NS, Dieguez-Lucena JL, Joensuu T, Lau S, Montgomery KT, Krahe R, et al. Mutations in KERA, encoding keratocan, cause cornea plana. Nat Genet 2000;25:91–5. 10.1038/75664. [DOI] [PubMed] [Google Scholar]
- [163].Chen J, Wong-Chong J, SundarRaj N. FGF-2– and TGF-β1–induced downregulation of lumican and keratocan in activated corneal keratocytes by JNK signaling pathway. Investig Opthalmol Visual Sci 2011;52:8957. 10.1167/iovs.11-8078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Lorenzo-Martín E, Gallego-Muñoz P, Mar S, Fernández I, Cidad P, Martínez-García MC. Dynamic changes of the extracellular matrix during corneal wound healing. Exp Eye Res 2019;186:107704. 10.1016/j.exer.2019.107704. [DOI] [PubMed] [Google Scholar]
- [165].Shami A, Gustafsson R, Kalamajski S, Krams R, Segers D, Rauch U, et al. Fibromodulin deficiency reduces low-density lipoprotein accumulation in atherosclerotic plaques in apolipoprotein E–null mice. Arterioscler Thromb Vasc Biol 2013;33:354–61. 10.1161/ATVBAHA.112.300723. [DOI] [PubMed] [Google Scholar]
- [166].Pourhanifeh MH, Mohammadi R, Noruzi S, Hosseini SA, Fanoudi S, Mohamadi Y, et al. The role of fibromodulin in cancer pathogenesis: implications for diagnosis and therapy. Cancer Cell Int 2019;19:157. 10.1186/s12935-019-0870-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Burton-Wurster N, Liu W, Matthews GL, Lust G, Roughley PJ, Glant TT, et al. TGF beta 1 and biglycan, decorin, and fibromodulin metabolism in canine cartilage. Osteoarthritis Cartilage 2003;11:167–76. 10.1053/S1063-4584(02)00349-7. [DOI] [PubMed] [Google Scholar]
- [168].Hultgårdh-Nilsson A, Borén J, Chakravarti S. The small leucine-rich repeat proteoglycans in tissue repair and atherosclerosis. J Intern Med 2015;278:447–61. 10.1111/joim.12400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Tasheva ES, Koester A, Paulsen AQ, Garrett AS, Boyle DL, Davidson HJ, et al. Mimecan/osteoglycin-deficient mice have collagen fibril abnormalities. Mol Vis 2002;8:407–15. [PubMed] [Google Scholar]
- [170].Mohan RR, Tovey J CK, Gupta R, Sharma A, Tandon A. Decorin biology, expression, function and therapy in the cornea. Curr Mol Med 2011;11:110–28. 10.2174/156652411794859241. [DOI] [PubMed] [Google Scholar]
- [171].Zhang G, Chen S, Goldoni S, Calder BW, Simpson HC, Owens RT, et al. Genetic evidence for the coordinated regulation of collagen fibrillogenesis in the cornea by decorin and biglycan. J Biol Chem 2009;284:8888–97. 10.1074/jbc.M806590200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Rødahl E, Van Ginderdeuren R, Knappskog PM, Bredrup C, Boman H. A second decorin frame shift mutation in a family with congenital stromal corneal dystrophy. Am J Ophthalmol 2006;142:520–1. 10.1016/j.ajo.2006.03.064. [DOI] [PubMed] [Google Scholar]
- [173].Chen S, Sun M, Meng X, Iozzo RV, Kao WW-Y, Birk DE. Pathophysiological mechanisms of autosomal dominant congenital stromal corneal dystrophy. Am J Pathol 2011;179:2409–19. 10.1016/j.ajpath.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Gubbiotti MA, Vallet SD, Ricard-Blum S, Iozzo RV. Decorin interacting network: a comprehensive analysis of decorin-binding partners and their versatile functions. Matrix Biol 2016;55:7–21. 10.1016/j.matbio.2016.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Yamaguchi Y, Mann DM, Ruoslahti E. Negative regulation of transforming growth factor-β by the proteoglycan decorin. Nature 1990;346:281–4. 10.1038/346281a0. [DOI] [PubMed] [Google Scholar]
- [176].Hildebrand A, Romarís M, Rasmussen LM, Heinegård D, Twardzik DR, Border WA, et al. Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor β. Biochem J 1994;302:527–34. 10.1042/bj3020527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Kou I, Nakajima M, Ikegawa S. Binding characteristics of the osteoarthritis-associated protein asporin. J Bone Miner Metabol 2010;28:395–402. 10.1007/s00774-009-0145-8. [DOI] [PubMed] [Google Scholar]
- [178].Horiguchi M, Ota M, Rifkin DB. Matrix control of transforming growth factor-function. J Biochem 2012;152:321–9. 10.1093/jb/mvs089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Niimori D, Kawano R, Felemban A, Niimori-Kita K, Tanaka H, Ihn H, et al. Tsukushi controls the hair cycle by regulating TGF-β1 signaling. Dev Biol 2012;372:81–7. 10.1016/j.ydbio.2012.08.030. [DOI] [PubMed] [Google Scholar]
- [180].Karamichos D, Hutcheon AEK, Zieske JD. Reversal of fibrosis by TGF-β3 in a 3D in vitro model. Exp Eye Res 2014;124:31–6. 10.1016/j.exer.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Funderburgh JL, Hevelone ND, Roth MR, Funderburgh ML, Rodrigues MR, Nirankari VS, et al. Decorin and biglycan of normal and pathologic human corneas. Invest Ophthalmol Vis Sci 1998;39:1957–64. [PubMed] [Google Scholar]
- [182].Bleckmann H, Schnoy N, Kresse H. Electron microscopic and immunohistochemical examination of scarred human cornea re-treated by excimer laser. Graefe’s Arch Clin Exp Ophthalmol 2002;240:271–8. 10.1007/s00417-002-0430-x. [DOI] [PubMed] [Google Scholar]
- [183].Mohan RR, Gupta R, Mehan MK, Cowden JW, Sinha S. Decorin transfection suppresses profibrogenic genes and myofibroblast formation in human corneal fibroblasts. Exp Eye Res 2010;91:238–45. 10.1016/j.exer.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Mohan RR, Tandon A, Sharma A, Cowden JW, Tovey JCK. Significant inhibition of corneal scarring in vivo with tissue-selective, targeted AAV5 decorin gene therapy. Investig Opthalmol Visual Sci 2011;52:4833. 10.1167/iovs.11-7357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Mohan RR, Tovey JCK, Sharma A, Schultz GS, Cowden JW, Tandon A. Targeted decorin gene therapy delivered with adeno-associated virus effectively retards corneal neovascularization in vivo. PLoS One 2011;6:e26432. 10.1371/journal.pone.0026432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Young MF, Bi Y, Ameye L, Chen X-D. Biglycan knockout mice: new models for musculoskeletal diseases. Glycoconj J 2002;19:257–62. 10.1023/A:1025336114352. [DOI] [PubMed] [Google Scholar]
- [187].Andrlova H, Mastroianni J, Madl J, Kern JS, Melchinger W, Dierbach H, et al. ´ Biglycan expression in the melanoma microenvironment promotes invasiveness via increased tissue stiffness inducing integrin-β1 expression. Oncotarget 2017;8:42901–16. 10.18632/oncotarget.17160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Mutoji KN, Sun M, Elliott G, Moreno IY, Hughes C, Gesteira TF, et al. Extracellular matrix deposition and remodeling after corneal alkali burn in mice. Int J Mol Sci 2021;22:5708. 10.3390/ijms22115708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Hayashi Y, Liu C-Y, Jester JJ, Hayashi M, Wang I-J, Funderburgh JL, et al. Excess biglycan causes eyelid malformation by perturbing muscle development and TGF-α signaling. Dev Biol 2005;277:222–34. 10.1016/j.ydbio.2004.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Liu C-Y, Olsen BR, Kao WW-Y. Developmental patterns of two α1(IX) collagen mRNA isoforms in mouse. Dev Dynam 1993;198:150–7. 10.1002/aja.1001980208. [DOI] [PubMed] [Google Scholar]
- [191].Pattwell DM, Sheridan CM, Le Goff M, Bishop PN, Hiscott P. Localisation of opticin in human proliferative retinal disease. Exp Eye Res 2010;90:461–4. 10.1016/j.exer.2009.12.007. [DOI] [PubMed] [Google Scholar]
- [192].Sugars RV, Olsson M-L, Marchner S, Hultenby K, Wendel M. The glycosylation profile of osteoadherin alters during endochondral bone formation. Bone 2013;53:459–67. 10.1016/j.bone.2013.01.022. [DOI] [PubMed] [Google Scholar]
- [193].Nikdin H, Olsson M-L, Hultenby K, Sugars RV. Osteoadherin accumulates in the predentin towards the mineralization front in the developing tooth. PLoS One 2012;7:e31525. 10.1371/journal.pone.0031525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Heinegård D, Larsson T, Sommarin Y, Franzen A, Paulsson M, Hedbom E. Two ´ novel matrix proteins isolated from articular cartilage show wide distributions among connective tissues. J Biol Chem 1986;261:13866–72. 10.1016/S0021-9258(18)67101-7. [DOI] [PubMed] [Google Scholar]
- [195].Heinegård D. Fell-Muir Lecture: proteoglycans and more - from molecules to biology. Int J Exp Pathol 2009;90:575–86. 10.1111/j.1365-2613.2009.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Birke MT, Lipo E, Adhi M, Birke K, Kumar-Singh R. AAV-mediated expression of human PRELP inhibits complement activation, choroidal neovascularization and deposition of membrane attack complex in mice. Gene Ther 2014;21:507–13. 10.1038/gt.2014.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Hughes RM, Simons BW, Khan H, Miller R, Kugler V, Torquato S, et al. Asporin restricts mesenchymal stromal cell differentiation, alters the tumor microenvironment, and drives metastatic progression. Cancer Res 2019;79:3636–50. 10.1158/0008-5472.CAN-18-2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Mecham RP, editor. The extracellular matrix: an overview. Berlin, Heidelberg: Springer Berlin Heidelberg; 2011. 10.1007/978-3-642-16555-9. [DOI] [Google Scholar]
- [199].Sharif R, Fowler B, Karamichos D. Collagen cross-linking impact on keratoconus extracellular matrix. PLoS One 2018;13:e0200704. 10.1371/journal.pone.0200704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Pusch CM, Zeitz C, Brandau O, Pesch K, Achatz H, Feil S, et al. The complete form of X-linked congenital stationary night blindness is caused by mutations in a gene encoding a leucine-rich repeat protein. Nat Genet 2000;26:324–7. 10.1038/81627. [DOI] [PubMed] [Google Scholar]
- [201].Bech-Hansen NT, Naylor MJ, Maybaum TA, Sparkes RL, Koop B, Birch DG, et al. Mutations in NYX, encoding the leucine-rich proteoglycan nyctalopin, cause X-linked complete congenital stationary night blindness. Nat Genet 2000;26:319–23. 10.1038/81619. [DOI] [PubMed] [Google Scholar]
- [202].Nishiu J, Tanaka T, Nakamura Y. Identification of a novel gene (ECM2) encoding a putative extracellular matrix protein expressed predominantly in adipose and female-specific tissues and its chromosomal localization to 9q22.3. Genomics 1998;52:378–81. 10.1006/geno.1998.5455. [DOI] [PubMed] [Google Scholar]
- [203].Neill T, Schaefer L, Iozzo RV. Decorin. Am J Pathol 2012;181:380–7. 10.1016/j.ajpath.2012.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Yan H, Chen Y, Li L, Jiang J, Wu G, Zuo Y, et al. Decorin alleviated chronic hydrocephalus via inhibiting TGF-β1/Smad/CTGF pathway after subarachnoid hemorrhage in rats. Brain Res 2016;1630:241–53. 10.1016/j.brainres.2015.11.004. [DOI] [PubMed] [Google Scholar]
- [205].Chaudhary K, Moore H, Tandon A, Gupta S, Khanna R, Mohan RR. Nanotechnology and adeno-associated virus-based decorin gene therapy ameliorates peritoneal fibrosis. Am J Physiol Ren Physiol 2014;307:F777–82. 10.1152/ajprenal.00653.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Zhang Z, Wu F, Zheng F, Li H. Adenovirus-mediated decorin gene transfection has therapeutic effects in a streptozocin-induced diabetic rat model. Nephron Exp Nephrol 2010;116:e11–21. 10.1159/000314669. [DOI] [PubMed] [Google Scholar]
- [207].Shen Y, Russo V, Zeglinski MR, Sellers SL, Wu Z, Oram C, et al. Recombinant decorin fusion protein attenuates murine abdominal aortic aneurysm formation and rupture. Sci Rep 2017;7:15857. 10.1038/s41598-017-16194-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Mao L, Yang J, Yue J, Chen Y, Zhou H, Fan D, et al. Decorin deficiency promotes epithelial-mesenchymal transition and colon cancer metastasis. Matrix Biol 2021;95:1–14. 10.1016/j.matbio.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Kolb M, Margetts PJ, Galt T, Sime PJ, Xing Z, Schmidt M, et al. Transient transgene expression of decorin in the lung reduces the fibrotic response to bleomycin. Am J Respir Crit Care Med 2001;163:770–7. 10.1164/ajrccm.163.3.2006084. [DOI] [PubMed] [Google Scholar]
- [210].Schneider M, Dillinger AE, Ohlmann A, Iozzo RV, Fuchshofer R. Decorin—an antagonist of TGF-β in astrocytes of the optic nerve. Int J Mol Sci 2021;22:7660. 10.3390/ijms22147660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Dodd Sayani, Shen Nedelec, Tredget Ghahary, et al. Delayed appearance of decorin in healing burn scars. Histopathology 2000;36:262–72. 10.1046/j.1365-2559.2000.00824.x. [DOI] [PubMed] [Google Scholar]
- [212].Dale P, DevoreBruce H, DewoolfsonVance Thompson. Composition and methods for the prevention and treatment of macular degeneration, diabetic retinopathy, and diabetic macular edema. 2010.
- [213].Kathleen Cogan FarinasSteven M. Chamow. Sustained drug delivery system. 2009.
- [214].Xie C, Mondal DK, Ulas M, Neill T, Iozzo RV. Oncosuppressive roles of decorin through regulation of multiple receptors and diverse signaling pathways. Am J Physiol Cell Physiol 2022;322:C554–66. 10.1152/ajpcell.00016.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Li Y, Hong J, Oh J-E, Yoon A-R, Yun C-O. Potent antitumor effect of tumor microenvironment-targeted oncolytic adenovirus against desmoplastic pancreatic cancer. Int J Cancer 2018;142:392–413. 10.1002/ijc.31060. [DOI] [PubMed] [Google Scholar]
- [216].Reed CC, Waterhouse A, Kirby S, Kay P, Owens RT, McQuillan DJ, et al. Decorin prevents metastatic spreading of breast cancer. Oncogene 2005;24:1104–10. 10.1038/sj.onc.1208329. [DOI] [PubMed] [Google Scholar]
- [217].Guilherme Tralhão J, Schaefer L, Micegova M, Evaristo C, Schönherr E, Kayal S, et al. In vivo selective and distant killing of cancer cells, using adenovirus-mediated decorin gene transfer. Faseb J 2003;17:1–21. 10.1096/fj.02-0534fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Santra M, Mann DM, Mercer EW, Skorski T, Calabretta B, Iozzo RV. Ectopic expression of decorin protein core causes a generalized growth suppression in neoplastic cells of various histogenetic origin and requires endogenous p21, an inhibitor of cyclin-dependent kinases. J Clin Invest 1997;100:149–57. 10.1172/JCI119507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Järveläinen H, Sainio A, Wight TN. Pivotal role for decorin in angiogenesis. Matrix Biol 2015;43:15–26. 10.1016/j.matbio.2015.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Nastase MV, Janicova A, Roedig H, Hsieh LT-H, Wygrecka M, Schaefer L. Small leucine-rich proteoglycans in renal inflammation: two sides of the coin. J Histochem Cytochem 2018;66:261–72. 10.1369/0022155417738752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Grisanti S, Szurman P, Warga M, Kaczmarek R, Ziemssen F, Tatar O, et al. Decorin modulates wound healing in experimental glaucoma filtration surgery: a pilot study. Investig Opthalmol Visual Sci 2005;46:191. 10.1167/iovs.04-0902. [DOI] [PubMed] [Google Scholar]
- [222].Ahmed Z, Bansal D, Tizzard K, Surey S, Esmaeili M, Gonzalez AM, et al. Decorin blocks scarring and cystic cavitation in acute and induces scar dissolution in chronic spinal cord wounds. Neurobiol Dis 2014;64:163–76. 10.1016/j.nbd.2013.12.008. [DOI] [PubMed] [Google Scholar]
- [223].Sainio AO, Järveläinen HT. Decorin-mediated oncosuppression - a potential future adjuvant therapy for human epithelial cancers. Br J Pharmacol 2019;176:5–15. 10.1111/bph.14180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Bahl N, Stone G, McLean M, Ho KKY, Birzniece V. Decorin, a growth hormone-regulated protein in humans. Eur J Endocrinol 2018;178:145–52. 10.1530/EJE-17-0844. [DOI] [PubMed] [Google Scholar]
- [225].Kamil S, Mohan RR. Corneal stromal wound healing: major regulators and therapeutic targets. Ocul Surf 2021;19:290–306. 10.1016/j.jtos.2020.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Zhang W, Ge Y, Cheng Q, Zhang Q, Fang L, Zheng J. Decorin is a pivotal effector in the extracellular matrix and tumour microenvironment. Oncotarget 2018;9:5480–91. 10.18632/oncotarget.23869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Hill LJ, Moakes RJA, Vareechon C, Butt G, Ng A, Brock K, et al. Sustained release of decorin to the surface of the eye enables scarless corneal regeneration. NPJ Regen Med 2018;3:23. 10.1038/s41536-018-0061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Chouhan G, Moakes RJA, Esmaeili M, Hill LJ, deCogan F, Hardwicke J, et al. A self-healing hydrogel eye drop for the sustained delivery of decorin to prevent corneal scarring. Biomaterials 2019;210:41–50. 10.1016/j.biomaterials.2019.04.013. [DOI] [PubMed] [Google Scholar]
- [229].Roberts CJ, Metzler KM, Mahmoud AM, Liu J. Biomechanical evaluation of response to treatment with human decorin core protein in ex-vivo human and porcine corneas. Invest Ophthalmol Vis Sci 2015;56:1104. [Google Scholar]
- [230].Sinjab MM, Cummings AB, editors. Corneal collagen cross linking. Cham: Springer International Publishing; 2017. 10.1007/978-3-319-39775-7. [DOI] [Google Scholar]
- [231].The DeScar Trial - The scar free foundation [n.d].
- [232].Ashish Tandon ASJRRM. Molecular mechanism of corneal neovascularization inhibition by decorin therapy. Invest Ophthalmol Vis Sci 2013;54:2082. [Google Scholar]
- [233].Mohan RR, Balne PK, Muayad MS, Tripathi R, Sinha NR, Gupta S, et al. Six-month in vivo safety profiling of topical ocular AAV5–decorin gene transfer. Transl Vis Sci Technol 2021;10:5. 10.1167/tvst.10.10.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].Ständer M, Naumann U, Dumitrescu L, Heneka M, Löschmann P, Gulbins E, et al. Decorin gene transfer-mediated suppression of TGF-β synthesis abrogates experimental malignant glioma growth in vivo. Gene Ther 1998;5:1187–94. 10.1038/sj.gt.3300709. [DOI] [PubMed] [Google Scholar]
- [235].Ma H-I, Hueng D-Y, Shui H-A, Han J-M, Wang C-H, Lai Y-H, et al. Intratumoral decorin gene delivery by AAV vector inhibits brain glioblastomas and prolongs survival of animals by inducing cell differentiation. Int J Mol Sci 2014;15:4393–414. 10.3390/ijms15034393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Isaka Y, Brees DK, Ikegaya K, Kaneda Y, Imai E, Noble NA, et al. Gene therapy by skeletal muscle expression of decorin prevents fibrotic disease in rat kidney. Nat Med 1996;2:418–23. 10.1038/nm0496-418. [DOI] [PubMed] [Google Scholar]
- [237].Sanon S. Effect of adenovirus-mediated decorin gene therapy on fibrotic remodeling in tissue-engineered skin. 2016.
- [238].Mohan RR, Tripathi R, Sharma A, Sinha PR, Giuliano EA, Hesemann NP, et al. Decorin antagonizes corneal fibroblast migration via caveolae-mediated endocytosis of epidermal growth factor receptor. Exp Eye Res 2019;180:200–7. 10.1016/j.exer.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Mohan Wmsssmvn RR. Decorin gene transfer into keratocytes inhibits alpha smooth muscle actin (SMA) expression and myofibroblast transformation. Invest Ophthalmol Vis Sci 2006;47:1815. [Google Scholar]
- [240].Donnelly KS, Giuliano EA, Sharma A, Tandon A, Rodier JT, Mohan RR. Decorin-PEI nanoconstruct attenuates equine corneal fibroblast differentiation. Vet Ophthalmol 2014;17:162–9. 10.1111/vop.12060. [DOI] [PubMed] [Google Scholar]
- [241].AmentaAtilgan YilmazBeth Alison R, Fallon A McKechnieJustin R. Therapeutic and diagnostic methods involving biglycan and utrophin. 2011.
- [242].Justin R. FallonMark A. BoweBeth McKechnieMichael RafiiAlison AmentaMary lynn MercadoHiroki hagiwara. Biglycan and related therapeutics and methods of use. 2000. [Google Scholar]
- [243].Fallon JR, McNally EM. Non-glycanated biglycan and LTBP4: leveraging the extracellular matrix for Duchenne muscular dystrophy therapeutics. Matrix Biol 2018;68–69:616–27. 10.1016/j.matbio.2018.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [244].Justin R. FallonBeth MckechnieAlison Rosalind AmentaMary Lynn Mercado. Therapeutic compositions containing modified class i slrp proteins. 2007. [Google Scholar]
- [245].Babelova A, Moreth K, Tsalastra-Greul W, Zeng-Brouwers J, Eickelberg O, Young MF, et al. Biglycan, a danger signal that activates the NLRP3 inflammasome via toll-like and P2X receptors. J Biol Chem 2009;284:24035–48. 10.1074/jbc.M109.014266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246].Zhao S, Yin X, Zhao W, Liu L, Wang Z. Biglycan as a potential diagnostic and prognostic biomarker in multiple human cancers. Oncol Lett 2020. 10.3892/ol.2020.11266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [247].Pechanec MY, Boyd TN, Baar K, Mienaltowski MJ. Adding exogenous biglycan or decorin improves tendon formation for equine peritenon and tendon proper cells in vitro. BMC Muscoskel Disord 2020;21:627. 10.1186/s12891-020-03650-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [248].Wang W, Chapman NM, Zhang B, Li M, Fan M, Laribee RN, et al. Upregulation of PD-L1 via HMGB1-activated IRF3 and NF-κB contributes to UV radiation-induced immune suppression. Cancer Res 2019;79:2909–22. 10.1158/0008-5472.CAN-18-3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [249].Cong L, Maishi N, Annan DA, Young MF, Morimoto H, Morimoto M, et al. Inhibition of stromal biglycan promotes normalization of the tumor microenvironment and enhances chemotherapeutic efficacy. Breast Cancer Res 2021;23:51. 10.1186/s13058-021-01423-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [250].Zheng S, Zou Y, Tang Y, Yang A, Liang J-Y, Wu L, et al. Landscape of cancer-associated fibroblasts identifies the secreted biglycan as a protumor and immunosuppressive factor in triple-negative breast cancer. OncoImmunology 2022;11:2020984. 10.1080/2162402X.2021.2020984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [251].Giatagana E-M, Berdiaki A, Gaardløs M, Tsatsakis AM, Samsonov SA, Nikitovic D. Rapamycin-induced autophagy in osteosarcoma cells is mediated via the biglycan/Wnt/β-catenin signaling axis. Am J Physiol Cell Physiol 2022;323:C1740–56. 10.1152/ajpcell.00368.2022. [DOI] [PubMed] [Google Scholar]
- [252].Hosseini F, Alemi F, Malakoti F, Mahmoodpoor A, Younesi S, Yousefi B, et al. Targeting Wnt/β-catenin signaling by microRNAs as a therapeutic approach in chemoresistant osteosarcoma. Biochem Pharmacol 2021;193:114758. 10.1016/j.bcp.2021.114758. [DOI] [PubMed] [Google Scholar]
- [253].Lucas-Martin A, Foz-Sala M, Todd I, Bottazzo GF, Pujol-Borrell R. Occurrence of thyrocyte HLA class II expression in a wide variety of thyroid diseases: relationship with lymphocytic infiltration and thyroid autoantibodies. J Clin Endocrinol Metab 1988;66:367–75. 10.1210/jcem-66-2-367. [DOI] [PubMed] [Google Scholar]
- [254].Miklós SÁNTHASzilvia GondaErika BereczkiPéter FERDINANDYTamás CSONT. Use of biglycan or enhancers of biglycan activity in the preparation of pharmaceutical compositions. 2007.
- [255].Diehl V, Huber LS, Trebicka J, Wygrecka M, Iozzo RV, Schaefer L. The role of decorin and biglycan signaling in tumorigenesis. Front Oncol 2021;11:801801. 10.3389/fonc.2021.801801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [256].Manupati K, Paul R, Hao M, Haas M, Bian ZC, Holm TM, et al. Biglycan promotes cancer stem cell properties, NFκB signaling and metastatic potential in breast cancer cells. Cancers 2022;14. 10.3390/cancers14020455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [257].Sun H, Wang X, Zhang Y, Che X, Liu Z, Zhang L, et al. Biglycan enhances the ability of migration and invasion in endometrial cancer. Arch Gynecol Obstet 2016;293:429–38. 10.1007/s00404-015-3844-5. [DOI] [PubMed] [Google Scholar]
- [258].Gopinath P, Natarajan A, Sathyanarayanan A, Veluswami S, Gopisetty G. The multifaceted role of Matricellular Proteins in health and cancer, as biomarkers and therapeutic targets. Gene 2022;815:146137. 10.1016/j.gene.2021.146137. [DOI] [PubMed] [Google Scholar]
- [259].Morimoto H, Hida Y, Maishi N, Nishihara H, Hatanaka Y, Li C, et al. Biglycan, tumor endothelial cell secreting proteoglycan, as possible biomarker for lung cancer. Thorac Cancer 2021;12:1347–57. 10.1111/1759-7714.13907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [260].Wiener Hus-Thehren U-HKSBDEAGAHGHHCPMD. Use of biglycan in the assessment of heart failure. 2009.
- [261].Hyunsoo Kim Eksk. Pharmaceutical composition for preventing or treating obesity containing biglycan as active ingredient. 2020.
- [262].Hyunsoo Kim. Composition for preventing or treating diabetes comprising biglycan. 2019.
- [263].Erkki I RuoslahtiWayne A Border. Use of decorin or biglycan for the manufacture of a medicament in the treatment of diabetes-associated pathology. 1992.
- [264].Erkki I RuoslahtiWayne A Border. Methods of preventing or reducing scarring with decorin or biglycan. 1994.
- [265].Li X, Truty MA, Kang Y, Chopin-Laly X, Zhang R, Roife D, et al. Extracellular lumican inhibits pancreatic cancer cell growth and is associated with prolonged survival after surgery. Clin Cancer Res 2014;20:6529–40. 10.1158/1078-0432.CCR-14-0970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [266].Li X, Truty MA, Kang Y, Chopin-Laly X, Zhang R, Roife D, et al. Extracellular lumican inhibits pancreatic cancer cell growth and is associated with prolonged survival after surgery. Clin Cancer Res 2014;20:6529–40. 10.1158/1078-0432.CCR-14-0970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [267].Yang C-T, Hsu P-C, Chow S-E. Downregulation of lumican enhanced mitotic defects and aneuploidy in lung cancer cells. Cell Cycle 2020;19:97–108. 10.1080/15384101.2019.1693189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [268].Giatagana E-M, Berdiaki A, Tsatsakis A, Tzanakakis GN, Nikitovic D. Lumican in carcinogenesis-revisited. Biomolecules 2021;11. 10.3390/biom11091319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [269].Kang Y, Roife D, Lee Y, Lv H, Suzuki R, Ling J, et al. Transforming growth factor-β limits secretion of lumican by activated stellate cells within primary pancreatic adenocarcinoma tumors. Clin Cancer Res 2016;22:4934–46. 10.1158/1078-0432.CCR-15-2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [270].Nikitovic D, Chalkiadaki G, Berdiaki A, Aggelidakis J, Katonis P, Karamanos NK, et al. Lumican regulates osteosarcoma cell adhesion by modulating TGFβ2 activity. Int J Biochem Cell Biol 2011;43:928–35. 10.1016/j.biocel.2011.03.008. [DOI] [PubMed] [Google Scholar]
- [271].Zeltz C, Brézillon S, Käpylä J, Eble JA, Bobichon H, Terryn C, et al. Lumican inhibits cell migration through α2β1 integrin. Exp Cell Res 2010;316:2922–31. 10.1016/j.yexcr.2010.08.002. [DOI] [PubMed] [Google Scholar]
- [272].Chen L, Zhang Y, Zuo Y, Ma F, Song H. Lumican expression in gastric cancer and its association with biological behavior and prognosis. Oncol Lett 2017. 10.3892/ol.2017.6842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [273].Ho TH, Serie DJ, Parasramka M, Cheville JC, Bot BM, Tan W, et al. Differential gene expression profiling of matched primary renal cell carcinoma and metastases reveals upregulation of extracellular matrix genes. Ann Oncol 2017;28:604–10. 10.1093/annonc/mdw652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [274].Maiti G, Frikeche J, Lam CY-M, Biswas A, Shinde V, Samanovic M, et al. Matrix lumican endocytosed by immune cells controls receptor ligand trafficking to promote TLR4 and restrict TLR9 in sepsis. Proc Natl Acad Sci U S A 2021;118. 10.1073/pnas.2100999118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [275].Yam GH-F, Yusoff NZBM, Kadaba A, Tian D, Myint HH, Beuerman RW, et al. Ex vivo propagation of human corneal stromal “activated keratocytes” for tissue engineering. Cell Transplant 2015;24:1845–61. 10.3727/096368914X685069. [DOI] [PubMed] [Google Scholar]
- [276].Amjadi S, Mai K, McCluskey P, Wakefield D. The role of lumican in ocular disease. ISRN Ophthalmol 2013;2013:1–7. 10.1155/2013/632302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [277].Zeltz C, Brézillon S, Käpylä J, Eble JA, Bobichon H, Terryn C, et al. Lumican inhibits cell migration through α2β1 integrin. Exp Cell Res 2010;316:2922–31. 10.1016/j.yexcr.2010.08.002. [DOI] [PubMed] [Google Scholar]
- [278].Stasiak M, Boncela J, Perreau C, Karamanou K, Chatron-Colliet A, Proult I, et al. Lumican inhibits SNAIL-induced melanoma cell migration specifically by blocking MMP-14 activity. PLoS One 2016;11:e0150226. 10.1371/journal.pone.0150226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [279].Chakravarti S, Petroll WM, Hassell JR, Jester JV, Lass JH, Paul J, et al. Corneal opacity in lumican-null mice: defects in collagen fibril structure and packing in the posterior stroma. Invest Ophthalmol Vis Sci 2000;41:3365–73. [PMC free article] [PubMed] [Google Scholar]
- [280].Brezillon S, Zeltz C, Schneider L, Terryn C, Vuillermoz B, Ramont L, et al. Lumican inhibits B16F1 melanoma cell lung metastasis. J Physiol Pharmacol 2009;60 (Suppl 4):15–22. [PubMed] [Google Scholar]
- [281].Xu X, Zhang Y, Ha P, Chen Y, Li C, Yen E, et al. A novel injectable fibromodulin-releasing granular hydrogel for tendon healing and functional recovery. Bioeng Transl Med 2023;8. 10.1002/btm2.10355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [282].Yang P, Li C, Lee M, Marzvanyan A, Zhao Z, Ting K, et al. Photopolymerizable hydrogel-encapsulated fibromodulin-reprogrammed cells for muscle regeneration. Tissue Eng 2020;26:1112–22. 10.1089/ten.tea.2020.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [283].Halasi M, Grinstein M, Adini A, Adini I. Fibromodulin ablation exacerbates the severity of acute colitis. J Inflamm Res 2022;15:4515–26. 10.2147/JIR.S366290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [284].Mormone E, Lu Y, Ge X, Fiel MI, Nieto N. Fibromodulin, an oxidative stress-sensitive proteoglycan, regulates the fibrogenic response to liver injury in mice. Gastroenterology 2012;142:612–621.e5. 10.1053/j.gastro.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [285].Zheng Z, Zhang X, Dang C, Beanes S, Chang GX, Chen Y, et al. Fibromodulin is essential for fetal-type scarless cutaneous wound healing. Am J Pathol 2016;186:2824–32. 10.1016/j.ajpath.2016.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [286].Zheng Z, James AW, Li C, Jiang W, Wang JZ, Chang GX, et al. Fibromodulin reduces scar formation in adult cutaneous wounds by eliciting a fetal-like phenotype. Signal Transduct Targeted Ther 2017;2:17050. 10.1038/sigtrans.2017.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [287].Xu X, Zhang Y, Ha P, Chen Y, Li C, Yen E, et al. A novel injectable fibromodulin-releasing granular hydrogel for tendon healing and functional recovery. Bioeng Transl Med 2023;8:e10355. 10.1002/btm2.10355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [288].Deng T, Hou Y, Lin G, Feng C, Liu K, Chen W, et al. A novel fibromodulin antagonist peptide RP4 exerts antitumor effects on colorectal cancer. Pharmaceutics 2023;15. 10.3390/pharmaceutics15030944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [289].Chia Soo. Fibromodulin formulation for reducing corneal scarring. 2009.
- [290].Chia SooKang TingZhong Zheng B. Methods of promoting angiogenesis in tissue with fibromodulin. 2015.
- [291].Cai X-B, Shen S-R, Chen D-F, Zhang Q, Jin Z-B. An overview of myopia genetics. Exp Eye Res 2019;188:107778. 10.1016/j.exer.2019.107778. [DOI] [PubMed] [Google Scholar]
- [292].Yip SP, Li CC, Yiu WC, Hung WH, Lam WW, Lai MC, et al. A novel missense mutation in the NYX gene associated with high myopia. Ophthalmic Physiol Opt 2013;33:346–53. 10.1111/opo.12036. [DOI] [PubMed] [Google Scholar]
- [293].Zhou L, Li T, Song X, Li Y, Li H, Dan H. NYX mutations in four families with high myopia with or without CSNB1. Mol Vis 2015;21:213–23. [PMC free article] [PubMed] [Google Scholar]
- [294].Zhang Q, Xiao X, Li S, Jia X, Yang Z, Huang S, et al. Mutations in NYX of individuals with high myopia, but without night blindness. Mol Vis 2007;13:330–6. [PMC free article] [PubMed] [Google Scholar]
- [295].Wang P, Li S, Xiao X, Guo X, Zhang Q. An evaluation of OPTC and EPYC as candidate genes for high myopia. Mol Vis 2009;15:2045–9. [PMC free article] [PubMed] [Google Scholar]
- [296].Majava M, Bishop PN, Hägg P, Scott PG, Rice A, Inglehearn C, et al. Novel mutations in the small leucine-rich repeat protein/proteoglycan (SLRP) genes in high myopia. Hum Mutat 2007;28:336–44. 10.1002/humu.20444. [DOI] [PubMed] [Google Scholar]
- [297].Huang C, Sharma A, Thakur R, Rai D, Katiki M, Germano J de F, et al. Asporin, an extracellular matrix protein, is a beneficial regulator of cardiac remodeling. Matrix Biol 2022;110:40–59. 10.1016/j.matbio.2022.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [298].Blomme A, Cusumano P, Peulen O, Bellahcene A, Castronovo V, Turtoi A. L’asporine : une nouvelle défense naturelle contre le cancer du sein. Méd/Sci 2016;32:1019–22. 10.1051/medsci/20163211020. [DOI] [PubMed] [Google Scholar]
- [299].Maris P, Blomme A, Palacios AP, Costanza B, Bellahcène A, Bianchi E, et al. Asporin is a fibroblast-derived TGF-β1 inhibitor and a tumor suppressor associated with good prognosis in breast cancer. PLoS Med 2015;12:e1001871. 10.1371/journal.pmed.1001871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [300].Simkova D, Kharaishvili G, Slabakova E, Murray PG, Bouchal J. Glycoprotein asporin as a novel player in tumour microenvironment and cancer progression. Biomed Pap 2016;160:467–73. 10.5507/bp.2016.037. [DOI] [PubMed] [Google Scholar]
- [301].Zhan S, Li J, Ge W. Multifaceted roles of asporin in cancer: current understanding. Front Oncol 2019;9. 10.3389/fonc.2019.00948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [302].Yashiro M, Hasegawa T, Yamamoto Y, Tsujio G, Nishimura S, Sera T, et al. Asporin expression on stromal cells and/or cancer cells might Be A useful prognostic marker in patients with diffuse-type gastric cancer. Eur Surg Res 2021;62:53–60. 10.1159/000515458. [DOI] [PubMed] [Google Scholar]
- [303].Turtoi A, Musmeci D, Wang Y, Dumont B, Somja J, Bevilacqua G, et al. Identification of novel accessible proteins bearing diagnostic and therapeutic potential in human pancreatic ductal adenocarcinoma. J Proteome Res 2011;10:4302–13. 10.1021/pr200527z. [DOI] [PubMed] [Google Scholar]
- [304].Satoyoshi R, Kuriyama S, Aiba N, Yashiro M, Tanaka M. Asporin activates coordinated invasion of scirrhous gastric cancer and cancer-associated fibroblasts. Oncogene 2015;34:650–60. 10.1038/onc.2013.584. [DOI] [PubMed] [Google Scholar]
- [305].Karamanos NK, Theocharis AD, Piperigkou Z, Manou D, Passi A, Skandalis SS, et al. A guide to the composition and functions of the extracellular matrix. FEBS J 2021;288:6850–912. 10.1111/febs.15776. [DOI] [PubMed] [Google Scholar]
- [306].Bailey TL, Johnson J, Grant CE, Noble WS. The MEME suite. Nucleic Acids Res 2015;43:W39–49. 10.1093/nar/gkv416. [DOI] [PMC free article] [PubMed] [Google Scholar]


