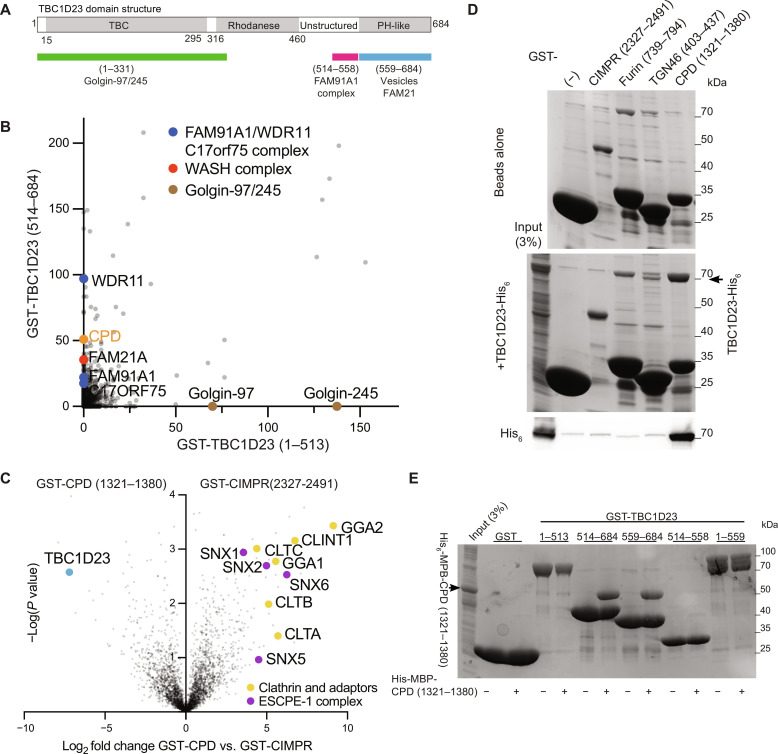
Fig. 1. The C-terminal domain of TBC1D23 forms a complex with the cytoplasmic tail of CPD.
(A) Domain structure of mouse TBC1D23 (UniProt Q8K0F1), the version of the protein used in this study. Labeling indicates the regions found by deletion mapping to bind the golgins, the FAM91A1 complex, the WASH complex, and vesicles (12, 14). (B) MS analysis from affinity chromatography of 293T cell lysates using GST-TBC1D23 fragments. The plot compares the average spectral counts from two independent replicates of GST-TBC1D23 (1 to 513) versus GST-TBC1D23 (514 to 684). Values are in data S1. (C) Volcano plot of MS analysis comparing the eluates from affinity chromatography of 293T cell lysates using the cytoplasmic tails of CPD or CIMPR. The plot compares the spectral intensities from proteins bound to each bait, using data from three independent biological replicates. Endosomal sorting complex for promoting exit 1 (ESCPE-1). Values are in data S1. (D) Coomassie-stained gel and anti-His6 immunoblot showing that TBC1D23-His6 binds directly and specifically to the cytoplasmic tail of CPD. GST-tagged tails of indicated endocytic cargoes were immobilized on beads and incubated with bacterial lysate containing TBC1D23-His6. Representative of three repeats. (E) Coomassie-stained gel showing that the C-terminal domain of TBC1D23 is necessary and sufficient for binding to CPD. GST-tagged fragments of TBC1D23 were immobilized on beads and incubated with lysate from bacteria expressing the cytoplasmic tail of CPD [His6-MBP-CPD (1321 to 1380)]. Representative of two repeats.