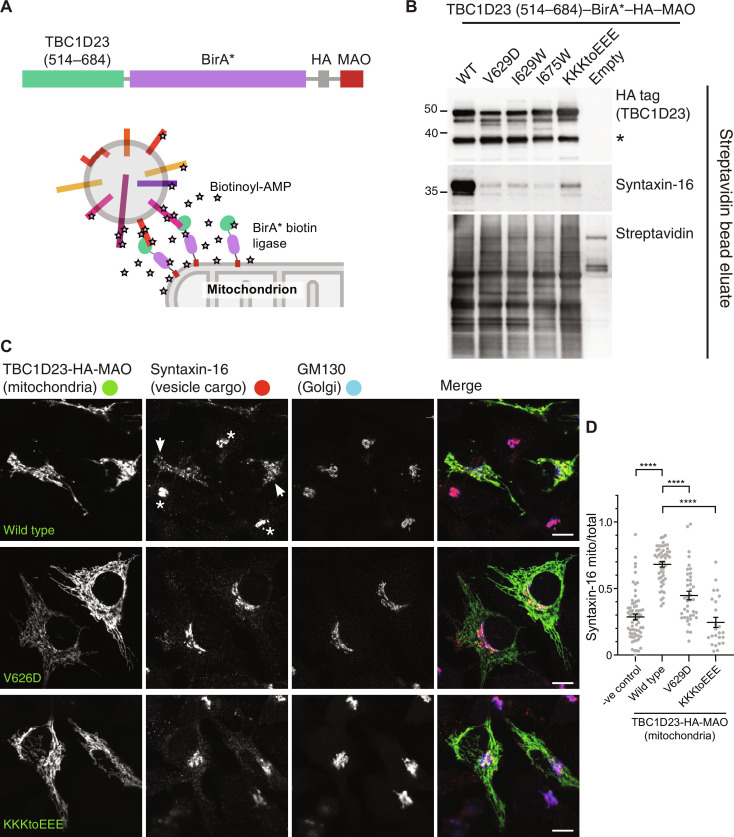
Fig. 7. Residues in TBC1D23 required for peptide binding in vitro are also required for vesicle capture in vivo.
(A) Ectopic relocation and biotinylation: a chimera comprising the TBC1D23 C-terminal domain attached to the BirA* promiscuous biotin ligase and the mitochondrial targeting signal from monoamine oxidase (MAO). (B) Immunoblots of streptavidin precipitations from whole-cell lysates of 293T cells transfected with the indicated variants of the mitochondrially targeted C-terminal domain of TBC1D23 fused to BirA*. Mutation of key residues does not affect total biotinylation or the stability of the chimera but greatly reduces biotinylation of syntaxin-16 indicating loss of vesicle capture. The lower band (asterisk) indicates some clipping of the chimera apparently between the TBC1D23 part and BirA*. Representative of three repeats. (C) Immunofluorescence of cells expressing the indicated forms of mitochondrial full-length TBC1D23 and immunolabeled for the TBC1D23 chimera [hemagglutinin (HA) tag], syntaxin-16, and the Golgi marker GM130. TBC1D23 is sufficient to cause the accumulation of vesicles at this ectopic location, and, hence, syntaxin-16 accumulates on mitochondria coated in wild-type TBC1D23 (arrows), rather than being predominantly in the Golgi in untransfected cells (asterisks). The mutations in TBC1D23 that disrupt peptide binding in vitro greatly reduce mitochondrial accumulation of syntaxin-16. Representative of three repeats. Scale bars, 10 μm. (D) Quantitation of the degree of mitochondrial relocation of syntaxin-16 in (C). The relocation induced by the wild-type protein, and the reductions in this relocation caused by the mutations are statistically significant (****P < 0.0001, unpaired, two-tailed t tests).