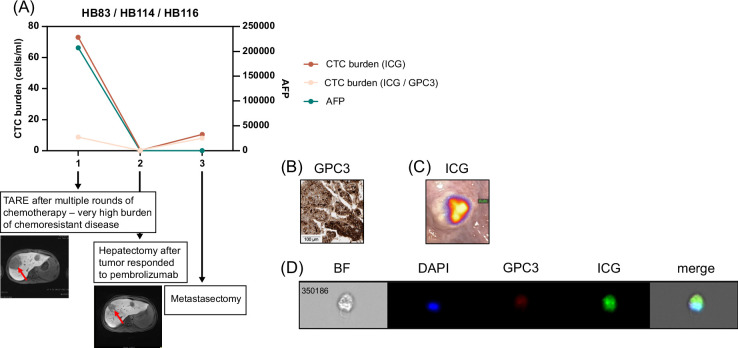
FIGURE 6.
CTC burden in patient HB83/114/116 with HCC. (A) We obtained samples at 3 time points during the patient’s course of treatment. We analyzed CTC burden after processing whole blood and tagging CTCs with ICG, GPC3, and DAPI, as described. We graphed CTC burden (cells/mL) and serum AFP levels, and both show a drop, correlating with the response of the patient to therapy. AFP was assessed by standard clinical tests. The DAPI+/ICG+/GPC3+ cell number shown is an average of counts by the standard and imaging flow cytometers for the HB83 sample. The DAPI+/ICG+ cell number shown was measured by the standard flow cytometer. CT images from the time of TARE and the time of hepatectomy with tumor (red arrows) decrease shown. (B, C) Validation of GPC3+ and ICG+ primary samples from patients. (B) Histology of primary patient tumor sample showing positivity of sample for GPC3. The scale bar represents 100 μm. (C) Near-infrared imaging of ICG+ lung nodule during metastasectomy. (D) Image of ICG+/GPC3+/DAPI+ CTC from Amnis ImageStream instrument. Abbreviations: AFP, alpha-fetoprotein; bf, brightfield; CTC, circulating tumor cell; GPC3, Glypican-3; ICG, indocyanine green; TARE, transarterial radioembolization.