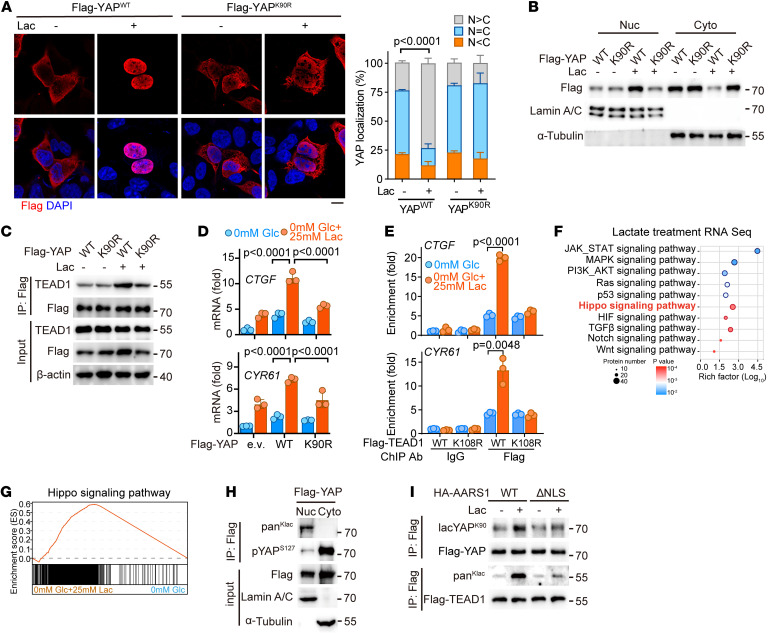
Figure 3. Lactylation promotes nuclear localization and stabilization of YAP-TEAD.
(A) Left: Immunofluorescence analysis using anti-FLAG antibody showing nuclear translocation of YAP in HEK293A cells transfected with FLAG-tagged YAP or its K90R mutant following lactate treatment. Right: The signal intensity of FLAG-YAP was quantified using ImageJ software (NIH) (n = 3). N, nuclear localization; C, cytosolic localization; Lac, lactate. Data are presented as mean ± SD. Scale bar: 5 μm. (B) Nucleocytoplasmic distribution of heterologously expressed YAP or its K90R mutant in lactate-treated cells. Nuc, nuclear localization; Cyto, cytosolic localization. (C) Coimmunoprecipitation analysis showing the interaction of YAP or its K90R mutant with TEAD1 in lactate-treated cells. (D) Real-time quantitative PCR (qPCR) showing the mRNA levels of CTGF and CYR61 in HEK293A cells overexpressing YAP or its K90R mutant following lactate treatment (n = 3). Data are presented as mean ± SD. Glc, glucose. (E) ChIP-qPCR analysis for the enrichment of TEAD1 or its K108R mutant on the indicated genes’ promoter in lactate-treated HEK293FT cells (n = 3). Data are presented as mean ± SD. (F) KEGG analysis of the differentially expressed genes in the glucose-deprived HGC27 cells with or without 25 mM lactate. (G) Gene set enrichment analysis of the Hippo pathway signature in the glucose-deprived HGC27 cells with or without 25 mM lactate. (H) Nucleocytoplasmic distribution of lactylation and phosphorylation of YAP in YAP-overexpressing HEK293FT cells. (I) Lactylation of exogenous YAP and TEAD1 in lactate-treated HEK293A cells transfected with AARS1 or its NLS-deletion (ΔNLS) mutant. Unpaired 2-tailed Student’s t test (A, D, and E).