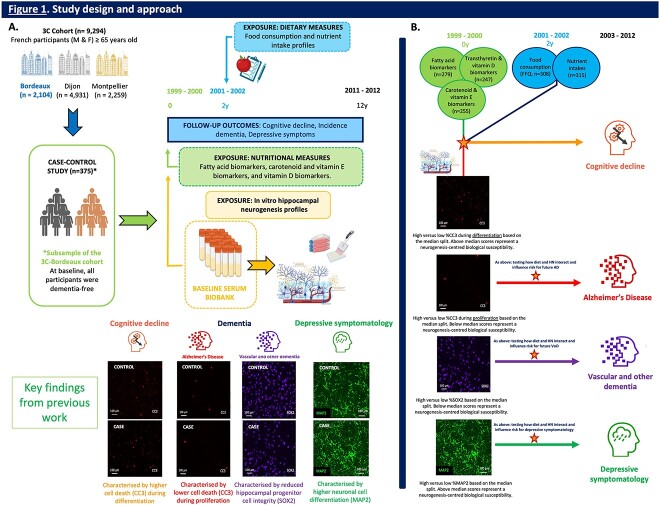
Figure 1.
Study design and approach. (A) Three City (3C) cohort and sample: Participants from the 3C study (n = 9,294) were recruited from three French cities: Bordeaux (n = 2,104), Dijon (n = 4,931) and Montpellier (n = 2,259) and specifically, a case–control study design on cognitive decline (CD) status (n = 375), nested within the 3C-Bordeaux cohort, was used for present analyses. Exposures: (i) Neurogenesis-centred biological susceptibility: Our previous work indicated that there may be a neurogenesis-centred biological susceptibility for CD, dementia, and depressive symptomatology that is already present up to 12 years prior to condition onset [9, 10]. Specifically, we found that increased baseline levels of cell death during differentiation (i.e., %CC3-d) increased the risk for future CD, whereas decreased baseline levels of cell death during proliferation (i.e., %CC3-p) increased the risk for future AD. Additionally, we found that reduced baseline levels of hippocampal progenitor cell integrity (i.e. %SOX2) increased the risk for VoD, whereas increased hippocampal cell differentiation (i.e., %MAP2) increased the risk for depressive symptomatology. Therefore, for present analyses, we categorised all participants with hippocampal neurogenesis (HN) profiles (n = 371), using a dichotomous classification approach (median split), focusing on biological susceptibility centred around these key HN readouts. (ii) Diet and nutrition: Data from three dietary/nutritional aspects were used to inform present analyses: (i) nutritional biomarker concentrations, including 12 fatty acids (n = 279), transthyretin and vitamin D (n = 247), and 6 carotenoid and 3 vitamin E biomarkers (n = 255), (ii) food consumption (in servings per week, n = 308), and (iii) macro- and micronutrient intakes (n = 315). Nutritional biomarker concentrations were measured in total plasma collected at baseline. Food consumption and nutrient intakes were determined by the Food Frequency Questionnaire (FFQ) and 24-h dietary recall, respectively, and were collected at the 2-year follow-up. (ii) Outcomes: (i) CD: Participants were classified as either having cognitive stability (control) or accelerated CD (case) based on their cognitive trajectories over 12 years. Cases had the worst slopes of CD across follow-up, whereas controls maintained cognitive function above the median slope. (ii) Dementia: At baseline, all participants were dementia-free. Over 12 years, dementia diagnosis was established by an independent committee of neurologists, following Diagnostic and Statistical Manual of Mental Disorders IV criteria. Dementia subtypes were consolidated into two primary categories for analysis, because of limited case numbers, which encompassed Alzheimer's Disease (AD) (i.e., probable/possible AD and mixed dementia) or VoD (i.e. vascular dementia, Parkinson dementia, Lewy body dementia, and frontotemporal dementia). (iii) Depressive symptomatology: Depressive symptomatology was assessed using the Center for Epidemiologic Studies Depression (CES-D) scale. Clinically relevant depressive symptoms at any assessment during the study duration were defined as scores ≥17 in men and ≥23 in women, or if participants were diagnosed with depression. (B) Overall approach: To determine whether diet and nutrition could influence the risk for future CD, dementia,and depressive symptomatology in participants with a neurogenesis-centred biological susceptibility relative to those without such a susceptibility, we tested the interaction between various dietary/nutritional factors and neurogenesis-centred biological susceptibility status, using multivariable-adjusted logistic regression models. Specifically, we tested for interactions between: (i) dietary/nutritional factors (i.e., food consumption, nutrient intakes, and nutritional biomarker concentrations) and high versus low levels of %CC3-d on CD, (ii) dietary/nutritional factors and high versus low levels of %CC3-p on AD, (iii) dietary/nutritional factors and high versus low levels of %SOX2 on VoD, and (iv) dietary/nutritional factors and high versus low levels of %MAP2 on depressive symptomatology. HN readout classification was dichotomously determined by median split. Abbreviations: M, male; F, female; y, years; h, hours; CC3 cleaved caspase 3; SOX2, SRY (sex determining region Y)-box 2; MAP2, microtubule-associated protein 2. Image created using BioRender software. (A) adapted from our previously published schematics on the cohort and experimental design [9, 10].