Abstract
Hypertensive patients with a higher proportion of genetic West African ancestry (%GWAA) have better blood pressure (BP) response to thiazide diuretics (TDs) and worse response to β‐blockers (BBs) than those with lower %GWAA, associated with their lower plasma renin activity (PRA). TDs and BBs are suggested to reduce BP in the long term through vasodilation via incompletely understood mechanisms. This study aimed at identifying pathways underlying ancestral differences in PRA, which might reflect pathways underlying BP‐lowering mechanisms of TDs and BBs. Among hypertensive participants enrolled in the Pharmacogenomics Evaluation of Antihypertensive Responses (PEAR) and PEAR‐2 trials, we previously identified 8 metabolites associated with baseline PRA and 4 metabolic clusters (including 39 metabolites) that are different between those with GWAA <45% versus ≥45%. In the current study, using Ingenuity Pathway Analysis (IPA), we integrated these signals. Three overlapping metabolic signals within three significantly enriched pathways were identified as associated with both PRA and %GWAA: ceramide signaling, sphingosine 1‐ phosphate signaling, and endothelial nitric oxide synthase signaling. Literature indicates that the identified pathways are involved in the regulation of the Rho kinase cascade, production of the vasoactive agents nitric oxide, prostacyclin, thromboxane A2, and endothelin 1; the pathways proposed to underlie TD‐ and BB‐induced vasodilatation. These findings may improve our understanding of the BP‐lowering mechanisms of TDs and BBs. This might provide a possible step forward in personalizing antihypertensive therapy by identifying patients expected to have robust BP‐lowering effects from these drugs.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Hypertensive patients with a higher proportion of genetic West African ancestry (%GWAA) have better blood pressure (BP) response to thiazide diuretics (TDs) and worse response to β‐blockers (BBs) than those with lower %GWAA. This ancestral differential BP response is associated with the lower plasma renin activity (PRA) of patients with a higher %GWAA. Additionally, TDs and BBs are suggested to lower BP in the long term through vasodilatation via incompletely understood mechanisms.
WHAT QUESTION DID THIS STUDY ADDRESS?
Do the pathways underlying ancestral differences in PRA reflect pathways underlying BP‐lowering mechanisms of TDs and BBs?
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
The integrated metabolomics work in this study shows that ceramide, S1P, and eNOS signaling pathways are commonly shared between our two sets of metabolomic signals associated with PRA and %GWAA. These pathways are suggested to be involved in the regulation of the Rho kinase cascade, production of the vasoactive agents nitric oxide, prostacyclin, thromboxane A2, and endothelin 1; and are pathways proposed to underlie TD‐ and BB‐induced vasodilatation.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
This study further elucidates the pathways underlying mechanisms of the BP‐lowering effects of the commonly prescribed TD and BB classes of antihypertensive drugs. This might help in personalizing antihypertensive therapy, and in developing antihypertensive drugs that target specific signaling pathways in patients with hypertension (HTN), which will ultimately improve BP control.
INTRODUCTION
Although most patients with hypertension (HTN) are prescribed more than one antihypertensive drug, only 30% of these patients have their blood pressure (BP) controlled. 1 Inter‐individual variability in BP response is likely a contributor to poorly controlled BP and is mainly a result of heterogeneity in pathophysiologic pathways underlying HTN. 1 Hypertensive patients of different racial/ancestral groups respond differently to antihypertensive drugs, especially to thiazide diuretics (TDs) and β‐blockers (BBs). Literature shows that genetically defined ancestry is a better predictor of BP response than self‐defined ethnicity. 2 Overall, hypertensive patients with a higher proportion of genetic West African ancestry (%GWAA) have a 3 mmHg greater systolic BP (SBP) lowering with TDs than those with lower %GWAA. In contrast, hypertensive patients with lower %GWAA have a 6 mmHg greater SBP reduction with BBs than those with higher %GWAA. 3
The observed racial/ancestral differences in BP response are primarily associated with the different plasma renin activity (PRA) levels between patients with higher %GWAA and those with lower %GWAA. 4 , 5 Measurement of PRA reflects the activity of the renin–angiotensin–aldosterone system (RAAS), an important BP regulator. Prior literature shows that high PRA (≥0.65 ng/mL/h) in most hypertensive patients with lower %GWAA is associated with renin‐dependent vasoconstriction HTN, which can be better controlled by BBs that decrease renal renin release. In contrast, low PRA (<0.65 ng/mL/h) in most hypertensive patients with higher %GWAA is associated with volume‐dependent HTN and salt sensitivity, which can be adequately controlled and corrected by antihypertensive drugs that enhance sodium excretion like TDs. 4 , 5 Additionally, several studies showed that PRA is a predictor of BP response to various antihypertensive drugs, including TDs and BBs. 5 , 6
We previously identified and replicated eight known metabolites associated with baseline PRA among two cohorts of European American hypertensive participants. 7 Additionally, we identified and replicated four metabolic clusters significantly different between hypertensive participants with lower versus higher %GWAA. 8 As PRA and %GWAA are key interconnected factors affecting BP response to TDs and BBs, we sought to identify the commonly shared canonical pathways between our metabolic signals associated with PRA and %GWAA using an integrated metabolomics analysis. We believe that identifying these pathways might give us novel mechanistic insights into the pathways underlying BP‐lowering mechanisms of TDs and BBs. A better understanding of these mechanisms can help personalize antihypertensive therapy and open new avenues for developing new antihypertensive drugs that target specific pathways, all leading to better BP control.
METHODS
The integration analysis was performed using Qiagen's Ingenuity Pathway Analysis (IPA QIAGEN Bioinformatics, CA, USA; https://digitalinsights.qiagen.com/IPA), utilizing the IPA metabolomics core analysis function, which identifies the significantly enriched canonical pathways (with a p < 0.05) using the right‐tailed Fisher's exact test. The null hypothesis of this test is that none of the metabolites included in the integration analysis overlap with any of the pathways present in the Ingenuity Knowledge Base. This knowledge base contains findings obtained from manually curated scientific literature, pathway and systems models, and public databases.
In this study, we imported the metabolomics dataset, including the Human Metabolome Database (HMDB) IDs of all the 47 metabolites associated with PRA and %GWAA phenotypes (Table S1). These 47 metabolic signals are organized into two sets, based on our previous work. The first set consists of all the replicated, known metabolites associated with baseline PRA in European American hypertensive participants (n = 8). 7 All eight metabolites, except gamma‐glutamylglutamine, are positively correlated with higher baseline PRA. The second set of metabolic signals consists of 39 known metabolites, which are included within the 4 replicated metabolic clusters significantly different between participants with lower versus higher %GWAA. These clusters are plasmalogens and lysoplasmalogens (n = 12 metabolites), sphingolipid metabolism and ceramides (n = 7 metabolites), cofactors and vitamins (n = 9 metabolites), and urea cycle–arginine–proline metabolism (n = 11 metabolites). 8 We hypothesized that the replicated metabolic signals, associated with PRA and %GWAA, share common canonical pathways. Using the IPA metabolomics core analysis function, we identified the significantly enriched canonical pathways (p < 0.05). Of these, the common pathways shared between metabolic signals associated with PRA and those associated with %GWAA were identified and presented. Further details on the methods as well as our prior metabolomics studies and signals are illustrated in the Supplement.
RESULTS
All 47 metabolites included in the analysis were mapped by the IPA. Using the IPA metabolomics core analysis, we identified three significantly enriched canonical pathways commonly shared between the metabolic signals associated with PRA and %GWAA: ceramide signaling (p = 1.15E−03), sphingosine 1‐phsophate (S1P) signaling (p = 1.15E−03), and endothelial nitric oxide synthase (eNOS) signaling (p = 1.38E−03) (Figure 1). The ceramide signaling and S1P signaling include S1P (associated with PRA) and stearoyl sphingomyelin (d18:1/18:0) (associated with %GWAA). The eNOS signaling includes S1P and arginine (associated with %GWAA).
FIGURE 1.
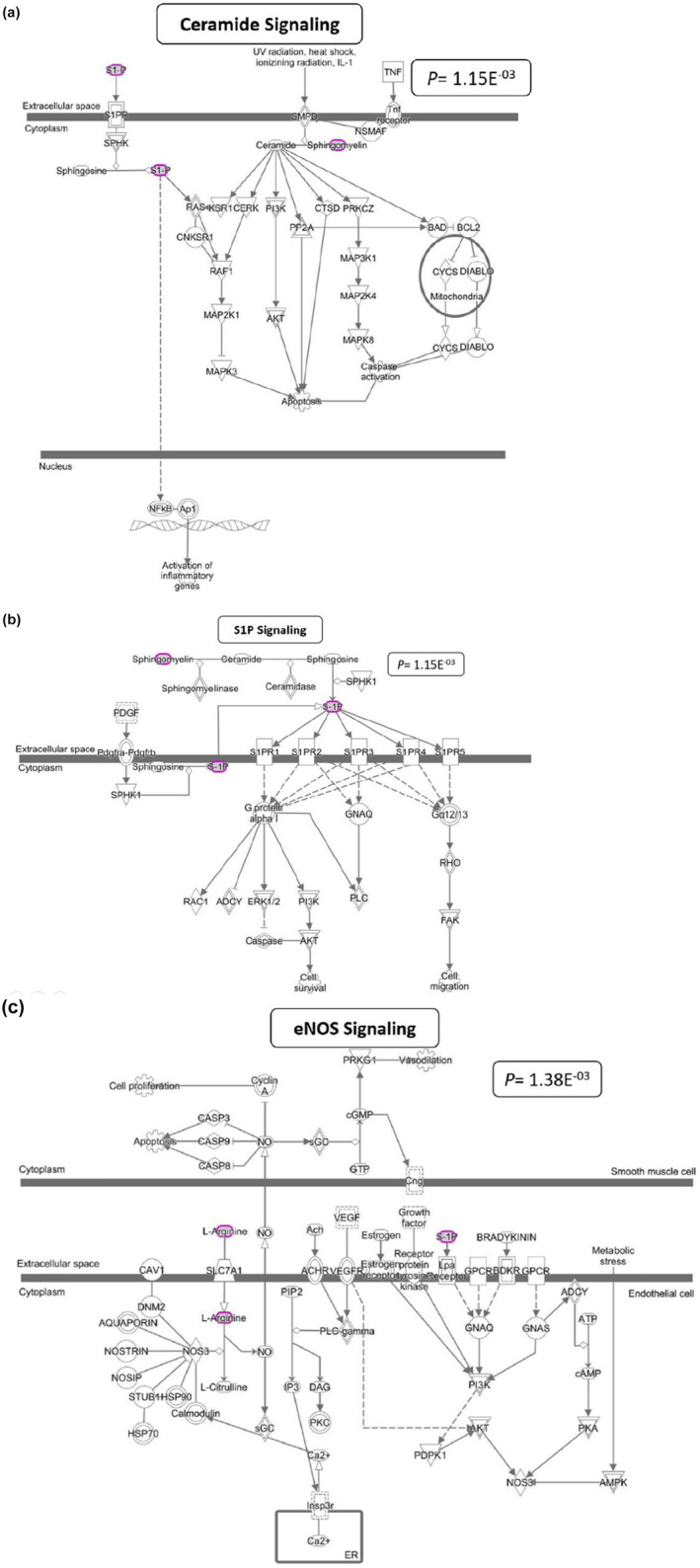
The three significantly enriched canonical pathways commonly shared between the metabolic signals associated with both PRA and %GWAA. These figures were obtained from the IPA tool. Molecules in purple circles are those metabolic signals, associated with either PRA or %GWAA phenotype. Ceramide signaling and S1P signaling pathways include S1P (associated with higher baseline PRA) and stearoyl sphingomyelin (d18:1/18:0) (more abundant in hypertensive participants with a higher vs lower %GWAA). The eNOS signaling pathway includes S1P (associated with higher baseline PRA) and arginine (more abundant in hypertensive participants with a higher vs lower %GWAA). IPA, Ingenuity Pathway Analysis; PRA, plasma renin activity; GWAA, genetic West African ancestry; S1P, sphingosine 1‐phosphate; eNOS, endothelial nitric oxide synthase.
As indicated in Figure 1a, within the ceramide signaling pathway, the second messenger ceramide induces apoptosis mainly by enhancing dephosphorylation of the pro‐apoptotic proteins: B‐cell lymphoma 2 (BCL2) and BCL2 antagonist of cell death (BAD).
As described in Figure 1b, within the S1P signaling pathway, S1P acts both extracellularly as a ligand for S1P receptors (S1PRs) and intracellularly as a second messenger. Activation of the S1PRs leads to either activation or inhibition of members of the Rho family of small GTPases, mainly Rho and Rac. Activated Rho stimulates the formation of contractile stress fibers, which play a role in vascular remodeling. Activated Rac stimulates the formation of the cortical actin network that plays a role in the activity of smooth muscle cells and vascular contractility.
As shown in Figure 1c, within the eNOS signaling pathway, the vasodilator nitric oxide (NO) is produced along with L‐citrulline via oxidation of L‐arginine. This reaction is catalyzed by the activity of the eNOS (NOS3) enzyme, which operates under the control of several second messengers. NO causes relaxation of the vascular smooth muscles through activation of soluble guanylyl cyclase (sGC) that leads to the production of cyclic guanosine monophosphate (cGMP) and dephosphorylation of myosin light chains. When the endothelial cells are exposed to the hemodynamic forces of blood, several signal transduction pathways are activated via G‐protein coupled receptors (GPCRs), leading to eNOS activation.
As indicated in Figure 2, the three canonical pathways are involved in several of the pathways proposed to underlie TD‐ and BB‐induced vasodilatation and long‐term BP reduction: regulation of Rho kinase cascade, 9 , 10 production of the vasodilators prostacyclin, 11 , 12 and NO, 12 , 13 , 14 and production of the vasoconstrictors thromboxane A2 15 and endothelin‐1. 16
FIGURE 2.
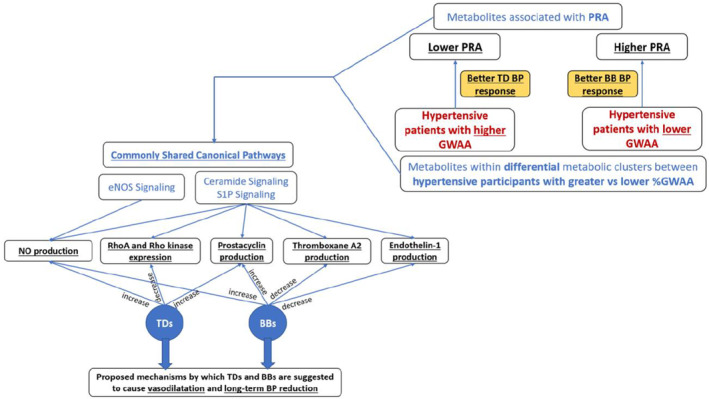
Connections between our integrated study findings and proposed mechanisms of TD‐ and BB‐induced direct vasodilatation and long‐term BP reduction. Our identified canonical pathways commonly shared between our metabolic signals associated with PRA and %GWAA are involved in the regulation of Rho kinase cascade, production of the vasodilators NO and prostacyclin, and production of the vasoconstrictors thromboxane A2, and endothelin‐1; the pathways proposed to underlie TD‐ and BB‐induced vasodilatation and long‐term BP reduction. GWAA, genetic West African ancestry; TD, thiazide diuretics; BB, β‐blockers; BP, blood pressure; PRA, plasma renin activity; S1P, sphingosine 1‐phosphate; eNOS, endothelial nitric oxide synthase.
DISCUSSION
In the current study, we integrated the metabolomic signals associated with baseline PRA and the metabolites included within each replicated ancestral metabolic cluster to further understand the pathways underlying BP‐lowering mechanisms of TDs and BBs. This integrated metabolomics work shows three significantly enriched canonical pathways commonly shared between our two sets of metabolomic signals: ceramide signaling, S1P signaling, and eNOS signaling.
As discussed below, prior studies indicate that these pathways might be involved in the proposed BP‐lowering mechanisms of TDs and BBs. Of note, the three identified pathways include our metabolic signal S1P (a bioactive sphingolipid), which is positively correlated with higher baseline/pre‐treatment PRA and poorer HCTZ BP response in our previous study. 7 Several prior studies also provided compelling evidence that S1P has a role in vascular tone, BP regulation, and HTN. S1P signaling induces different effects on the vasculature, depending on the S1P receptor subtypes: activation of the endothelial S1PR 1 and 3 leads to vasodilatation through production of the vasodilator NO, whereas stimulation of the smooth muscle S1PR 2 and 3 results in vasoconstriction via Rho kinase signaling. 17 , 18 The final outcome of S1P signaling can be either vasodilatation or vasoconstriction, mainly depending on the expression levels of the different S1PR subtypes. Moreover, it is hypothesized that S1PRs' expression and signaling are altered in patients with HTN. 18
TDs reduce BP mainly by increasing sodium excretion through inhibiting Na+/Cl‐ cotransporter in the kidneys. 19 BBs reduce BP mainly by reducing heart rate and cardiac output via blocking the action of catecholamines on the β1‐adrenergic receptors. 12 However, TD‐ and BB‐induced long‐term BP reduction is suggested to occur through vasodilatation via incompletely understood mechanisms. 12 , 19
One of the proposed mechanisms by which TDs might cause vasodilatation is through a reduction in the expression of the RhoA and Rho kinase proteins in the vascular smooth muscles, which leads to calcium desensitization. 10 Stimulating the S1PR 2 and 3 via S1P within our identified pathways ceramide signaling and S1P signaling leads to activation of the Rho family of small GTPases, mainly Rho, which results in the formation of the stress fibers. These actin‐based bundles cause vascular smooth muscle contraction, leading to vasoconstriction and increased BP. 20 Therefore, our data and previous data might support the proposed mechanism of TD‐induced vasodilatation through effects on the Rho kinase cascade and suggest that ceramide and S1P signaling pathways might be involved in this mechanism. The question remains whether TDs specifically induce long‐term vasodilation and BP reduction through inhibition of the RhoA signaling in hypertensive patients who have a predominant S1P signaling via S1PR 2 and 3.
TDs are also suggested to induce vasodilatation and long‐term BP reduction through increased production of the vasodilator prostacyclin. 21 Additionally, proposed mechanisms for the BB‐induced vasodilatation involve increased prostacyclin production 22 and decreased production of the vasoconstrictor thromboxane A2. 15 Sphingolipids, specifically sphingomyelin and ceramide, regulate the production of prostacyclin and thromboxane A2 through regulation of the phospholipase A2 enzymatic activity and arachidonic acid (the precursor of prostacyclin and thromboxane A2) release. Prior studies suggest that sphingomyelin inhibits the activity of phospholipase A2, whereas ceramide activates it. 23 Our findings highlight ceramide signaling and S1P signaling (both include S1P and stearoyl sphingomyelin (d18:1/18:0)) as pathways linked to both PRA and %GWAA phenotypes. Since PRA and %GWAA affect BP response to TDs and BBs, these findings might support the involvement of prostacyclin and thromboxane A2 in the TD‐ and BB‐induced vasodilation and long‐term BP reduction.
Prior studies demonstrate that BBs can reduce BP in long‐term through reduced synthesis and release of the vasopressor endothelin‐1. 16 Sphingolipids, specifically S1P, control endothelin‐1 release through increased intracellular calcium and decreased cyclic adenosine monophosphate (cAMP) production. 23 Our study identifies ceramide signaling and S1P signaling (both involve S1P) as commonly shared pathways between metabolic signals associated with PRA and %GWAA (the two interconnected factors that affect BB BP response). Therefore, these findings might support the suggested mechanism of BB‐induced BP‐lowering through decreased levels of endothelin‐1.
Another proposed mechanism for TD‐induced and BB‐induced direct vasodilatation involves the activation of eNOS and increased production of the vasorelaxant NO. 12 , 13 , 14 This mechanism is supported by our findings, suggesting that eNOS signaling, which includes our metabolic signals S1P and arginine, is a commonly shared pathway between metabolic signals, associated with PRA and %GWAA. S1P activates the S1PR 1 and 3, leading to the activation of eNOS through activation of phosphoinositide 3‐kinase and protein kinase B. 23 This mechanism has also been supported by prior pharmacometabolomic studies, which demonstrate that TD treatment is associated with increased levels of citrulline (a by‐product of NO production) from baseline. 24 , 25 Although higher S1P levels are associated with poorer BP response to HCTZ in our previous study, this might indicate that activation of S1P signaling via S1PR 2 and 3 led to vasoconstriction in the studied population.
In conclusion, our exploratory integrated metabolomics work sheds light on potential pathways that might support several proposed mechanisms underlying TD‐ and BB‐induced long‐term BP reduction. These mechanisms include reduced expression of the RhoA and Rho kinase proteins, increased production of the vasodilators prostacyclin and NO, and decreased production and release of the vasoconstrictors thromboxane A2 and endothelin‐1. These findings were generated using data from trial participants, all of whom were diagnosed with primary HTN without any cardiovascular disease or diabetes mellitus. Therefore, the pathways identified in this study might specifically reflect HTN and BP phenotypes. Future studies are warranted specifically to further study the expression levels of the different S1P receptors and S1P signaling in patients with HTN. Further understanding of the pathways underlying mechanisms of TD and BB BP‐lowering effects might help personalize antihypertensive therapy by matching the antihypertensive drug class that targets specific BP regulatory mechanism(s) in individual patients with HTN. These findings might also open new avenues for developing antihypertensive drugs that target specific signaling pathways in patients with HTN. This is anticipated to improve BP control and reduce the risk of cardiovascular diseases among these patients.
AUTHOR CONTRIBUTIONS
All authors wrote the manuscript. R.M.C.‐D. designed the research. M.M. performed the research. M.M. analyzed the data.
FUNDING INFORMATION
This study was funded by the National Institute of Health (NIH) Pharmacogenetics Research Network grant U01‐GM074492 and the National Center for Advancing Translational Sciences under the award number UL1 TR000064 (University of Florida), UL1 TR000454 (Emory University), and UL1 TR000135 (Mayo Clinic). The Pharmacogenomics Evaluation of Antihypertensive Responses efforts at Mayo Clinic were also supported by funds from Mayo Foundation.
CONFLICT OF INTEREST STATEMENT
The authors declared no competing interests for this work.
DISCLAIMER
The opinions expressed in this manuscript are those of the authors and should not be interpreted as the position of the US Food and Drug Administration.
Supporting information
Table S1.
Mehanna M, McDonough CW, Smith SM, et al. Integrated metabolomics analysis reveals mechanistic insights into variability in blood pressure response to thiazide diuretics and beta blockers. Clin Transl Sci. 2024;17:e13816. doi: 10.1111/cts.13816
Clinical Trial Registry Numbers: NCT01203852; NCT00246519.
REFERENCES
- 1. Cooper‐DeHoff RM, Fontil V, Carton T, et al. Tracking blood pressure control performance and process metrics in 25 US health systems: the PCORnet blood pressure control laboratory. J Am Heart Assoc. 2021;10:e022224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Iniesta R, Campbell D, Venturini C, et al. Gene variants at loci related to blood pressure account for variation in response to antihypertensive drugs between black and white individuals. Hypertension. 2019;74:614‐622. [DOI] [PubMed] [Google Scholar]
- 3. Sehgal AR. Overlap between whites and blacks in response to antihypertensive drugs. Hypertension. 2004;43:566‐572. [DOI] [PubMed] [Google Scholar]
- 4. Gharaibeh KA, Turner ST, Hamadah AM, et al. Comparison of blood pressure control rates among recommended drug selection strategies for initial therapy of hypertension. Am J Hypertens. 2016;29:1186‐1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mehanna M, Wang Z, Gong Y, et al. Plasma renin activity is a predictive biomarker of blood pressure response in European but not in African Americans with uncomplicated hypertension. Am J Hypertens. 2019;32:668‐675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Egan BM, Basile JN, Rehman SU, et al. Plasma renin test‐guided drug treatment algorithm for correcting patients with treated but uncontrolled hypertension: a randomized controlled trial. Am J Hypertens. 2009;22:792‐801. [DOI] [PubMed] [Google Scholar]
- 7. Mehanna M, McDonough CW, Smith SM, et al. Metabolomics signature of plasma renin activity and linkage with blood pressure response to Beta blockers and thiazide diuretics in hypertensive European American patients. Meta. 2021;11:645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mehanna M, McDonough CW, Smith SM, et al. Influence of genetic west African ancestry on metabolomics among hypertensive patients. Meta. 2022;12:783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhu Z, Zhu S, Liu D, Cao T, Wang L, Tepel M. Thiazide‐like diuretics attenuate agonist‐induced vasoconstriction by calcium desensitization linked to rho kinase. Hypertension. 2005;45:233‐239. [DOI] [PubMed] [Google Scholar]
- 10. Araos P, Mondaca D, Jalil JE, et al. Diuretics prevent rho‐kinase activation and expression of profibrotic/oxidative genes in the hypertensive aortic wall. Ther Adv Cardiovasc Dis. 2016;10:338‐347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Beckmann ML, Gerber JG, Byyny RL, LoVerde M, Nies AS. Propranolol increases prostacyclin synthesis in patients with essential hypertension. Hypertension. 1988;12:582‐588. [DOI] [PubMed] [Google Scholar]
- 12. Oliver E, Mayor F, D'Ocon P. Beta‐blockers: historical perspective and mechanisms of action. Rev Esp Cardiol (Engl Ed). 2019;72:853‐862. [DOI] [PubMed] [Google Scholar]
- 13. Sládková M, Kojsová S, Jendeková L, Pechánová O. Chronic and acute effects of different antihypertensive drugs on femoral artery relaxation of L‐NAME hypertensive rats. Physiol Res. 2007;56(Suppl 2):S85‐S91. [DOI] [PubMed] [Google Scholar]
- 14. Laurens C, Abot A, Delarue A, Knauf C. Central effects of Beta‐blockers may Be due to nitric oxide and hydrogen peroxide release independently of their ability to cross the blood‐brain barrier. Front Neurosci. 2019;13:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Viinikka L, Ylikorkala O. Beta blockers inhibit fetal thromboxane A2 production in vitro. Biol Neonate. 1988;54:169‐172. [DOI] [PubMed] [Google Scholar]
- 16. Brehm BR, Bertsch D, von Fallois J, Wolf SC. Beta‐blockers of the third generation inhibit endothelin‐1 liberation, mRNA production and proliferation of human coronary smooth muscle and endothelial cells. J Cardiovasc Pharmacol. 2000;36:S401‐S403. [DOI] [PubMed] [Google Scholar]
- 17. Jujic A, Matthes F, Vanherle L, et al. Plasma S1P (Sphingosine‐1‐phosphate) links to hypertension and biomarkers of inflammation and cardiovascular disease: findings from a translational investigation. Hypertension. 2021;78:195‐209. [DOI] [PubMed] [Google Scholar]
- 18. Katunaric B, SenthilKumar G, Schulz ME, De Oliveira N, Freed JK. S1P (Sphingosine‐1‐phosphate)‐induced vasodilation in human resistance arterioles during health and disease. Hypertension. 2022;79:2250‐2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rapoport RM, Soleimani M. Mechanism of thiazide diuretic arterial pressure reduction: the search continues. Front Pharmacol. 2019;10:815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang G, Yang L, Kim GS, et al. Critical role of sphingosine‐1‐phosphate receptor 2 (S1PR2) in acute vascular inflammation. Blood. 2013;122:443‐455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Uehara Y, Shirahase H, Nagata T, et al. Radical scavengers of indapamide in prostacyclin synthesis in rat smooth muscle cell. Hypertension. 1990;15:216‐224. [DOI] [PubMed] [Google Scholar]
- 22. Pettersson K, Hansson G, Björkman JA, Ablad B. Prostacyclin synthesis in relation to sympathoadrenal activation. Effects of beta‐blockade. Circulation. 1991;84:VI38‐VI43. [PubMed] [Google Scholar]
- 23. Spijkers LJ, Alewijnse AE, Peters SL. Sphingolipids and the orchestration of endothelium‐derived vasoactive factors: when endothelial function demands greasing. Mol Cells. 2010;29:105‐111. [DOI] [PubMed] [Google Scholar]
- 24. Altmaier E, Fobo G, Heier M, et al. Metabolomics approach reveals effects of antihypertensives and lipid‐lowering drugs on the human metabolism. Eur J Epidemiol. 2014;29:325‐336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hiltunen TP, Rimpelä JM, Mohney RP, Stirdivant SM, Kontula KK. Effects of four different antihypertensive drugs on plasma metabolomic profiles in patients with essential hypertension. PLoS One. 2017;12:e0187729. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.


