Table 1. Optimization for Catalytic Trimerization of Phenylacetylene.a.
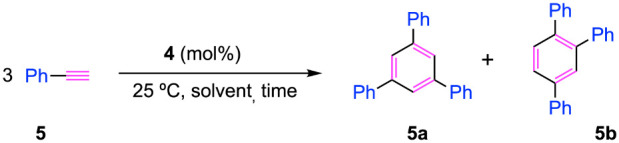
| entry | catalyst (mol %) | solvent | time (h) | yield (%)b | regioselectivity (5a:5b) |
|---|---|---|---|---|---|
| 1 | 4 (10) | C6D6 | 6 | >99 | 85:15 |
| 2 | 4 (10) | [D5]-pyridine | 6 | - | - |
| 3 | 4 (10) | [D3]- acetonitrile | 6 | - | - |
| 4 | 4 (7) | C6D6 | 6 | >99 | 85:15 |
| 5 | 4 (5) | C6D6 | 6 | >99 | 85:15 |
| 6 | 4 (2.5) | C6D6 | 6 | >99 | 85:15 |
| 7 | 4 (2.5) | C6D6 | 3 | >99 | 85:15 |
| 8 | 4 (2.5) | C6D6 | 3 | 90c | 93:7c |
| 9 | 2 (2.5) | C6D6 | 3 | - | - |
| 10 | 3 (2.5) | C6D6 | 3 | 7 | NDd |
Reaction conditions: phenylacetylene (0.3 mmol), 4 (10–2.5 mol %), 0.5 mL of benzene, room temperature, and 6–3 h.
Yields were determined by 1H NMR spectroscopy using a 10 mol % of ferrocene as internal standard.
Isolated yields: The isomer ratio of the isolated product was determined by 1H NMR spectroscopy.
The ratio could not be determined due to the low yield.
